Abstract
Human strains of Chlamydia trachomatis were inoculated unilaterally into the genital tracts of female TO, CBA, CBA/nu and C3H mice via the intrauterine route or under the ovarian bursa. Inflammatory changes were not seen in the oviducts or uterus of mice given two laboratory-adapted LGV serovars (L1 and L2), although chlamydiae were recovered from the lower genital tract. However, salpingitis and endometritis occurred after each of three chlamydial strains (serovars D and E) had been inoculated. Oviduct inflammation was seen for up to 6 weeks after inoculation but reached maximum severity usually after about 2 weeks, the lumen sometimes being occluded by exudate and necrotic debris. Pathological changes were seen often in both oviducts indicating canalicular spread of the organisms through the uterus. Pre-treatment of the mice with progesterone had an enhancing effect in that the lesions developed more rapidly; such treatment, in halting the oestrous cycle, probably made a larger number of target cells available for more efficient infection. Involvement of the oviduct on the uninoculated side occurred more rapidly in T-cell impaired nude mice than in immunologically normal mice, although there was little or no effect on the severity of the oviductal changes. There was evidence that the susceptibility of different strains of mice to chlamydial salpingitis varied. Thus, inflammatory changes in C3H mice were more severe than in TO mice and the changes in C3H and CBA strains were longer lasting than those in TO mice. This suggests that a possible genetic predisposition in the human situation should not be ignored. Finally, one chlamydial strain of low passage produced more severe salpingitis in mice than another strain of similar passage. By analogy different chlamydial strains may not be of equal pathogenicity in the human situation.
Full text
PDF





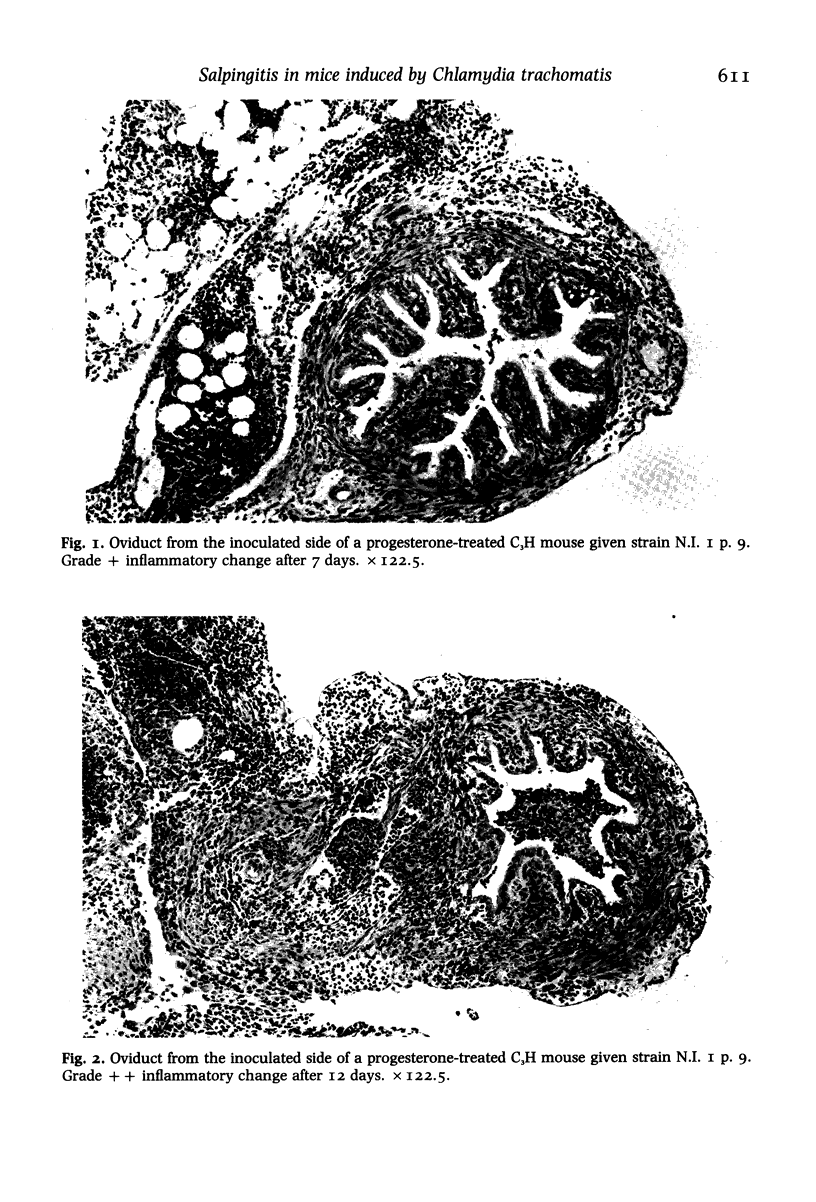
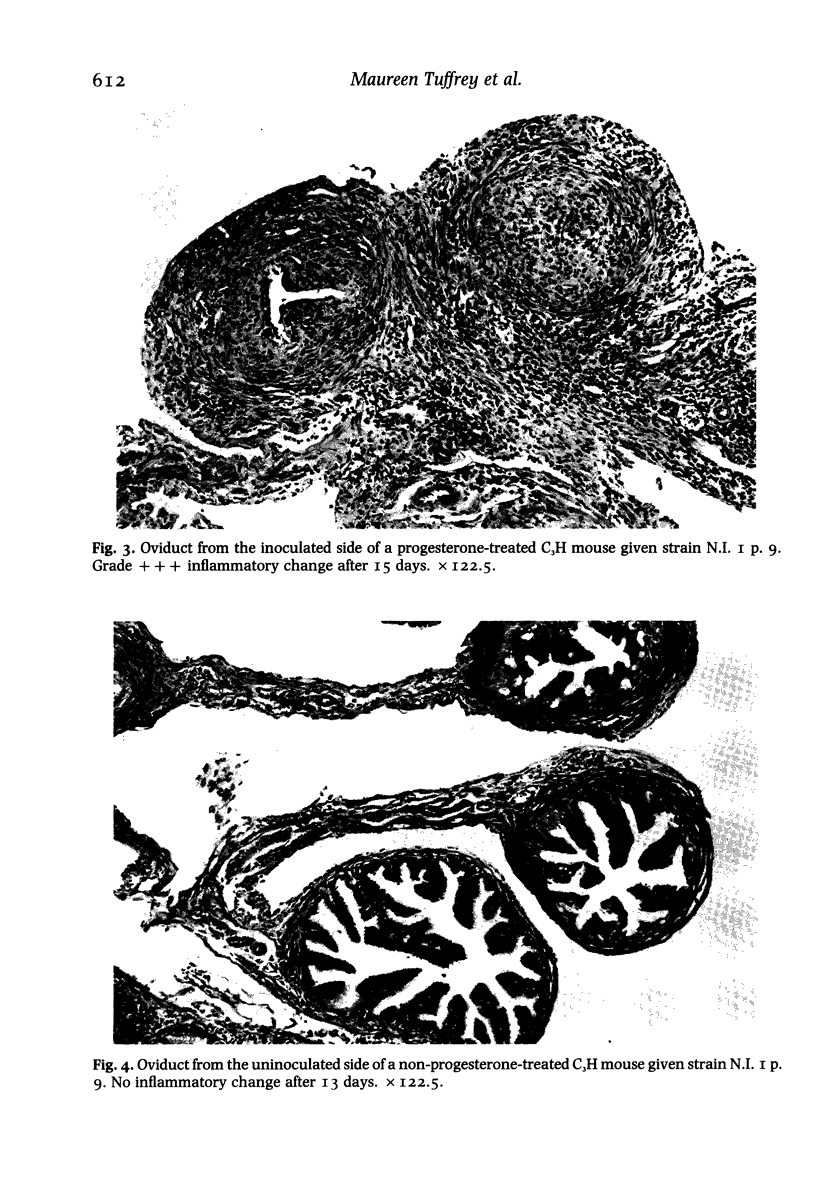




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Johnson A. P., Hare M. J., Wilbanks G. D., Cooper P., Hetherington C. M., Al-Kurdi M., Osborn M. F., Taylor-Robinson D. A colposcopic and histological study of experimental chlamydial cervicitis in marmosets. Br J Exp Pathol. 1984 Feb;65(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Møller B. R., Ingerselv H. J., Nüssler E., Weström L., Wølner-Hanssen P. Endometritis caused by Chlamydia trachomatis. Br J Vener Dis. 1981 Jun;57(3):191–195. doi: 10.1136/sti.57.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. R., Freundt E. A., Märdh P. A. Experimental pelvic inflammatory disease provoked by Chlamydia trachomatis and Mycoplasma hominis in grivet monkeys. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):990–995. doi: 10.1016/0002-9378(80)91094-7. [DOI] [PubMed] [Google Scholar]
- Patton D. L., Halbert S. A., Wang S. P. Experimental salpingitis in rabbits provoked by Chlamydia trachomatis. Fertil Steril. 1982 May;37(5):691–700. [PubMed] [Google Scholar]
- Sweet R. L. Chlamydial salpingitis and infertility. Fertil Steril. 1982 Nov;38(5):530–533. doi: 10.1016/s0015-0282(16)46629-x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Thomas B. J. The rôle of Chlamydia trachomatis in genital-tract and associated diseases. J Clin Pathol. 1980 Mar;33(3):205–233. doi: 10.1136/jcp.33.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Reeve P., Oriel J. D. Simplified serological test for antibodies to Chlamydia trachomatis. J Clin Microbiol. 1976 Jul;4(1):6–10. doi: 10.1128/jcm.4.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Falder P., Taylor-Robinson D. Effect on Chlamydia trachomatis infection of the murine genital tract of adoptive transfer of congenic immune cells or specific antibody. Br J Exp Pathol. 1985 Aug;66(4):427–433. [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Falder P., Taylor-Robinson D. Genital-tract infection and disease in nude and immunologically competent mice after inoculation of a human strain of Chlamydia trachomatis. Br J Exp Pathol. 1982 Oct;63(5):539–546. [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]









