Abstract
A novel staining procedure for enumerating osteoclasts on neonatal mouse calvaria with the vital fluorescent dye acridine orange is described. It has the advantage over Barnicot's neutral-red method in that the nuclei and cytoplasm of the osteoclast are stained differentially. The osteopetrotic calvarium (mi/mi) has fewer multinucleate osteoclasts than its normal counterpart (mi/+) and they are differently distributed. The osteopetrotic calvarium has more mononucleate cells which stain like osteoclasts with acridine orange than the normal calvarium and these cells also are differently distributed. These mononuclear cells may be mononuclear osteoclasts or their precursors. These observations suggest that the defect resulting in this osteopetrosis lies with osteoclast differentiation.
Full text
PDF





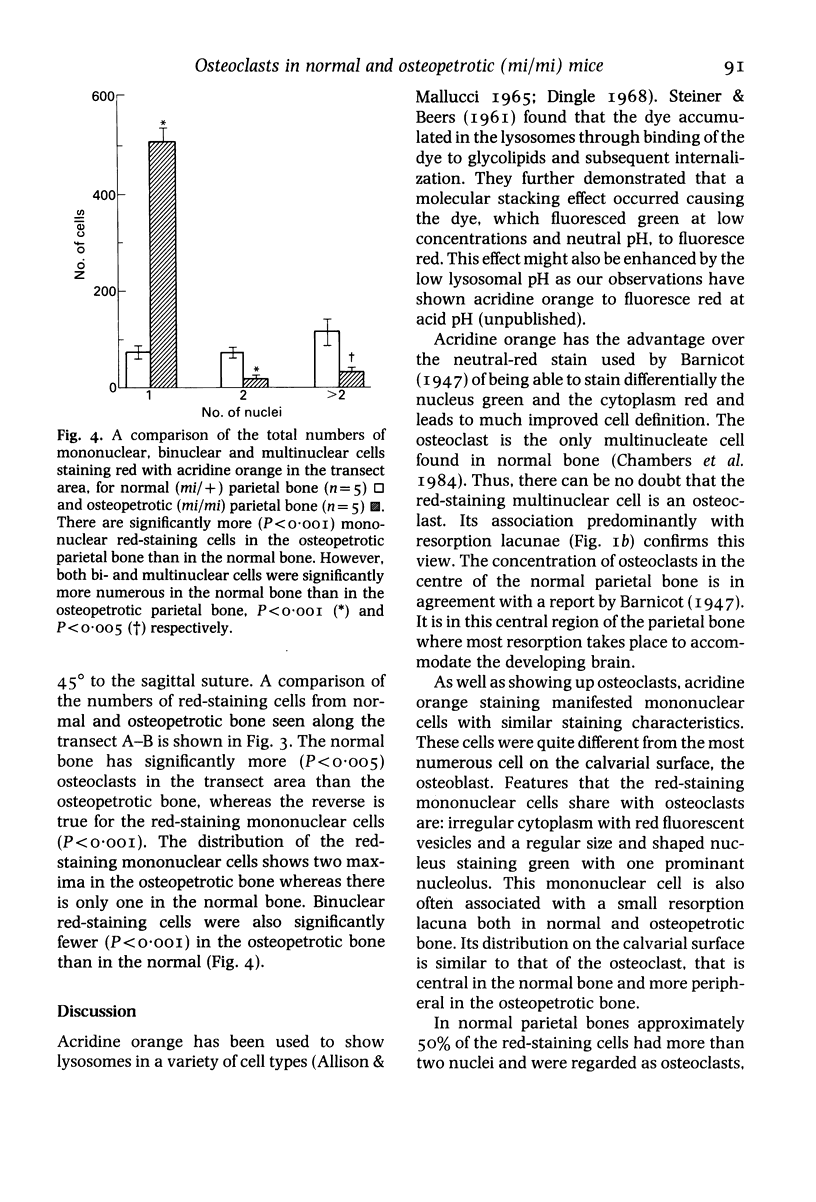


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K., Kanno T., Schneider G. B. Surface structures and osteoclasts of mouse parietal bones: a light and scanning electron microscopic study. Arch Histol Jpn. 1983 Dec;46(5):663–676. doi: 10.1679/aohc.46.663. [DOI] [PubMed] [Google Scholar]
- Ash P., Loutit J. F., Townsend K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980 Feb 14;283(5748):669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- CAMERON D. A. The fine structure of bone and calcified cartilage. A critical review of the contribution of electron microscopy to the understading of osteogenesis. Clin Orthop Relat Res. 1963;26:199–228. [PubMed] [Google Scholar]
- Cowden R. R., Curtis S. K. Acridine orange as a supravital fluorochrome indicating varying degrees of chromatin condensation. Histochemistry. 1974;40(4):305–310. doi: 10.1007/BF00495037. [DOI] [PubMed] [Google Scholar]
- Dingle J. T. Vacuoles, vesicles and lysosomes. Br Med Bull. 1968 May;24(2):141–145. doi: 10.1093/oxfordjournals.bmb.a070616. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr The origin of osteoclasts: evidence, clinical implications and investigative challenges of an extra-skeletal source. J Oral Pathol. 1983 Aug;12(4):226–256. doi: 10.1111/j.1600-0714.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Nisbet N. W., Menage J., Loutit J. F. Host-donor cellular interactions in the treatment of experimental osteopetrosis. Nature. 1978 Feb 2;271(5644):464–466. doi: 10.1038/271464a0. [DOI] [PubMed] [Google Scholar]
- Scott B. L. The occurrence of specific cytoplasmic granules in the osteoclast. J Ultrastruct Res. 1967 Aug 30;19(5):417–431. doi: 10.1016/s0022-5320(67)80071-6. [DOI] [PubMed] [Google Scholar]




