Abstract
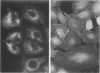
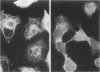
Hydrogen peroxide (H2O2) and other oxygen metabolites have been implicated in the pathogenesis of cell and tissue injury. The nature of the injury occurring in cells exposed to oxygen metabolites is unknown. A549 cells, derived from human lung carcinoma, were exposed to glucose-glucose oxidase or hydrogen peroxide in vitro. The distribution of actin and cytokeratin filaments, as well as 51chromium (51Cr) release and trypan blue dye exclusion were assessed. Both glucose-glucose oxidase and H2O2 resulted in changes which were time- and dose-dependent. Alterations in the cytoskeleton were detected by immunofluorescence microscopy at two hours, at which time the cells excluded trypan blue dye, while 51Cr release and trypan blue uptake first occurred at 8 h and required a five-fold greater concentration of glucose oxidase. The addition of catalase to glucose-glucose oxidase or H2O2, or inactivation of glucose oxidase by boiling, abrogated the injury. Therefore, one of the early targets of H2O2-induced cell injury may be the cytoskeleton.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Ceurremans S., Lehr I., Mahlmeister C., Paar E. Production of specific antibodies to contractile proteins, and their use in immunofluorescence microscopy. II. Species-specific and species-non-specific antibodies to smooth and striated chicken muscle actin. Histochemistry. 1977 Feb 1;50(4):271–279. doi: 10.1007/BF00507120. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Ley K., Arfors K. E. Changes in macromolecular permeability by intravascular generation of oxygen-derived free radicals. Microvasc Res. 1982 Jul;24(1):25–33. doi: 10.1016/0026-2862(82)90039-5. [DOI] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980 Jan 18;617(1):36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Nardone L. L., Andrews S. B. Cell line A549 as a model of the type II pneumocyte. Phospholipid biosynthesis from native and organometallic precursors. Biochim Biophys Acta. 1979 May 25;573(2):276–295. doi: 10.1016/0005-2760(79)90061-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Cohn Z. A. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981 Nov 1;154(5):1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J. Structural interaction of cytoskeletal components. J Cell Biol. 1981 Jul;90(1):222–235. doi: 10.1083/jcb.90.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman N. S., Wilton J. M. Leukotoxicity of an extract from Actinobacillus actinomycetemcomitans for human gingival polymorphonuclear leukocytes. Inflammation. 1981 Mar;5(1):1–12. doi: 10.1007/BF00910774. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The cytoskeleton. Natl Cancer Inst Monogr. 1982;60:31–46. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]