Abstract
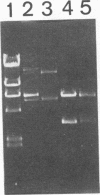
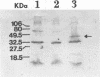
Heterologous expression of the Clostridium cellulovorans engB gene by Clostridium acetobutylicum BKW-1 was detected as zones of hydrolysis on carboxymethyl cellulose (CMC) Trypticase glucose yeast plates stained with Congo red. The extracellular cellulase preparation from C. acetobutylicum BKW-1 has a specific activity towards CMC which is more than fourfold that present in C. acetobutylicum ATCC 824. Western blot (immunoblot) analysis using the C. cellulovorans anti-EngB primary antibody demonstrated that an additional 44-kDa protein band was present in the supernatant derived from C. acetobutylicum BKW-1 but was not present in ATCC 824 or ATCC 824(pMTL500E).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Foong F. C., Doi R. H. Characterization and comparison of Clostridium cellulovorans endoglucanases-xylanases EngB and EngD hyperexpressed in Escherichia coli. J Bacteriol. 1992 Feb;174(4):1403–1409. doi: 10.1128/jb.174.4.1403-1409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong F., Hamamoto T., Shoseyov O., Doi R. H. Nucleotide sequence and characteristics of endoglucanase gene engB from Clostridium cellulovorans. J Gen Microbiol. 1991 Jul;137(7):1729–1736. doi: 10.1099/00221287-137-7-1729. [DOI] [PubMed] [Google Scholar]
- Gardner R. M., Doerner K. C., White B. A. Purification and characterization of an exo-beta-1,4-glucanase from Ruminococcus flavefaciens FD-1. J Bacteriol. 1987 Oct;169(10):4581–4588. doi: 10.1128/jb.169.10.4581-4588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. Y., Blaschek H. P. Construction and characterization of a phage-plasmid hybrid (phagemid), pCAK1, containing the replicative form of viruslike particle CAK1 isolated from Clostridium acetobutylicum NCIB 6444. J Bacteriol. 1993 Jun;175(12):3838–3843. doi: 10.1128/jb.175.12.3838-3843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. Y., Blaschek H. P. Construction of an Escherichia coli-Clostridium perfringens shuttle vector and plasmid transformation of Clostridium perfringens. Appl Environ Microbiol. 1989 Feb;55(2):360–365. doi: 10.1128/aem.55.2.360-365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. Y., Vertes A. A., Blaschek H. P. Isolation of a single-stranded plasmid from Clostridium acetobutylicum NCIB 6444. Appl Environ Microbiol. 1990 Jun;56(6):1725–1728. doi: 10.1128/aem.56.6.1725-1728.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Cellulolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Aug;50(2):220–228. doi: 10.1128/aem.50.2.220-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein L. D., Welker N. E., Bennett G. N., Papoutsakis E. T. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology (N Y) 1992 Feb;10(2):190–195. doi: 10.1038/nbt0292-190. [DOI] [PubMed] [Google Scholar]
- Shoseyov O., Doi R. H. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2192–2195. doi: 10.1073/pnas.87.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoseyov O., Hamamoto T., Foong F., Doi R. H. Cloning of Clostridium cellulovorans endo-1,4-beta-glucanase genes. Biochem Biophys Res Commun. 1990 Jun 15;169(2):667–672. doi: 10.1016/0006-291x(90)90382-w. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Rosenzweig S. A reliable method for the purification of bacteriophage lambda DNA. Biotechniques. 1989 Jul-Aug;7(7):694–696. [PubMed] [Google Scholar]
- Zappe H., Jones D. T., Woods D. R. Cloning and expression of Clostridium acetobutylicum endoglucanase, cellobiase and amino acid biosynthesis genes in Escherichia coli. J Gen Microbiol. 1986 May;132(5):1367–1372. doi: 10.1099/00221287-132-5-1367. [DOI] [PubMed] [Google Scholar]