Abstract
Antiestrogens are thought to exert most of their beneficial effects in breast cancer by antagonizing the actions of estrogen. We report here that antiestrogens also stimulate the expression of quinone reductase (QR) [NAD(P)H:quinone oxidoreductase, EC 1.6.99.2], which may provide protective effects against the toxicity and mutagenicity caused by quinones. QR is up-regulated by low concentrations of antiestrogens (trans-hydroxytamoxifen, tamoxifen, and ICI182,780) in estrogen receptor (ER)-containing breast cancer cells, and this increase is suppressed by estrogen via an ER-dependent mechanism. Since regulation of the QR gene, as well as other genes involved in detoxification such as the glutathione S-transferase Ya subunit (GST Ya) gene, is known to be mediated by an electrophile/antioxidant response element (EpRE/ARE), we examined the effects of antiestrogens on a 41-bp electrophile responsive region derived from the GST Ya gene. Transfection of this EpRE-containing region into ER-negative breast cancer cells in the presence or absence of an expression vector for the human ER, as well as mutagenesis studies, revealed that the EpRE-containing construct was activated by antiestrogen to the same extent as by tert-butylhydroquinone (TBHQ), a known activator of EpREs; however, only the stimulation by antiestrogen, and not TBHQ, required ER and was repressed by estradiol, although activation by both inducers mapped to the same 10-bp EpRE consensus sequence. Thus, there appear to be two pathways for QR induction, one that is activated by electrophile inducers such as TBHQ and is ER independent, and a second that is antiestrogen regulated and ER dependent; both pathways act through the EpRE. The anticancer action of antiestrogens may thus derive not only from the already well-known repression of estrogen-stimulated activities but also from the activation of detoxifying enzymes, such as QR, that may contribute to the beneficial antioxidant activity of antiestrogens.
Keywords: electrophile response element, tamoxifen, antioxidant, breast cancer
Tamoxifen is an anticancer drug that is widely used in the treatment of breast cancer (1–4). It is also being assessed as a preventive agent for this disease and for other potential benefits, such as protection against cardiovascular disease and osteoporosis (5, 6). It has been proposed that tamoxifen may function as an anticancer drug, possibly by acting as an antioxidant (7–9). However, the basis for the antioxidant capabilities of tamoxifen has not been well characterized.
Phase 2 detoxification enzymes such as NAD(P)H:(quinone-acceptor) oxidoreductase [quinone reductase (QR)], glutathione S-transferases (GSTs), epoxide hydrolase, and UDP-glucuronosyltransferases are induced in cells by electrophilic compounds and phenolic antioxidants (reviewed in refs. 10 and 11). These widely distributed enzymes detoxify electrophiles, thereby protecting cells against the toxic and neoplastic effects of carcinogens.
Using the technique of RNA differential display (12), we have identified in this report QR mRNA as a species that is expressed at much higher levels in an MCF-7 breast cancer cell subline that has been grown long term in the presence of the antiestrogen trans-hydroxytamoxifen (TOT) (13). We therefore undertook the examination of its regulation by antiestrogens as a possible basis for the proposed antioxidant action of tamoxifen. The molecular mechanisms for the induction of phase 2 enzymes by antiestrogen were also explored in view of their importance in devising strategies for chemoprotection against cancer. It has been reported that the induction of the GST Ya subunit (GST Ya) and QR genes is mediated through an electrophile (or antioxidant) response element (EpRE or ARE) (14, 15), although the identity of the EpRE/ARE enhancer binding protein(s) is not known.
In our studies, we have observed that the QR gene shows reversed pharmacology, being markedly up-regulated by antiestrogen and suppressed by estrogen in breast cancer cells. Our data indicate that there are two pathways for QR up-regulation, one that is antiestrogen modulated and estrogen receptor (ER) dependent, and a second that is stimulated by known electrophile inducers such as tert-butylhydroquinone (TBHQ) and is ER independent. Transfection and mutagenesis studies on gene constructs with the 41-bp EpRE-containing region from the GST Ya gene indicate that antiestrogen-mediated activation occurs at the transcriptional level via the EpRE and requires the ER. These observations have broad implications regarding potential antiestrogen regulation of a variety of genes whose transcription is under the control of EpRE/AREs.
MATERIALS AND METHODS
Chemicals and Materials.
Cell culture media were purchased from GIBCO. Calf serum was from HyClone and fetal calf serum (FCS) from Sigma. The antiestrogens ICI182,780 (ICI), tamoxifen, and TOT were kindly provided by Alan Wakeling and Zeneca Pharmaceuticals (Macclesfield, England). 12-Tetradecanoate 13-acetate (TPA), NADPH, menadione, and cytochrome c were obtained from Sigma. TBHQ was obtained from Aldrich.
Plasmids.
The growth hormone (GH) reporter gene constructs p284GstYa-GH (containing the mouse GST Ya gene minimal promoter region), p41–284GstYa-GH (containing the mouse GST Ya gene minimal promoter and the 5′ upstream 41-bp EpRE-containing region), and pTREX2–284GstYa-GH [containing the mouse GST Ya gene minimal promoter region and two consensus TPA response element (TRE) sites] were kindly provided by Paul Talalay (The John Hopkins University School of Medicine) (16).
Five reporter constructs, each containing mutations introduced sequentially into the 41-bp fragment containing the EpRE, were constructed by site-directed mutagenesis (17), with modification (18). The ScaI/SacI fragment of p41–284GstYa-GH was inserted into the SacI/SmaI site of Bluescript II SK+ (Stratagene) to make p41–284GstYa-BSK+. Mutagenic oligonucleotides were then annealed to single-stranded DNA generated using the f1 origin of replication in Bluescript II SK+. The mutagenic oligonucleotides used in five separate mutagenesis reactions were: 5′-CAGTGCCAAGCTCTGCAGAAAAATGACATTGC-3′, 5′-AGCTTAGCTTGGGTCCATGGTTGCTAATGGTG-3′, 5′-TTGGAAATGACATTGCTCTTCGTGACAAAGCA-3′, 5′-GACATTGCTAATTTGGATCCAGCAACTTTGTC-3′, and 5′-TAATGGTGACAAAGATCTTGTGTCGACTCTAG-3′. To make each of the five mutated p41–284GstYa-GH reporter constructs (M1–M5) the NdeI/SacI fragment of p41–284GstYa-GH was then replaced with the mutated NdeI/SacI fragment of p41–284GstYa-BSK+.
The expression vector for the wild-type human ER and the mutant ER that lacks activation function-2 activity (ERS554fs) has been described (19). The expression vector for the ER DNA binding mutant, ERDBDmut (missing amino acids 185–251), was constructed by replacing the EagI/EagI fragment from the wild-type pCMV-ER with the EagI/EagI insert from HE11, the latter plasmid kindly provided by Pierre Chambon (20). The plasmid pCMVβ (Clonetech, Palo Alto, CA), which encodes the β-galactosidase (β-gal) gene, was used as an internal control for transfection efficiency in all experiments.
Cell Culture.
MCF-7 human breast cancer cells were acquired from the Michigan Cancer Foundation. Cells were maintained in growth medium, which was minimum essential medium (MEM) plus phenol red supplemented with 5% heat-inactivated FCS, estradiol (E2) (10−12 M), and 10 mM Hepes. Prior to the experiments, cells were depleted of estrogen by growth for 2 weeks in the same growth medium except without added E2. MCF-7 cells were then grown in MEM plus phenol red supplemented with 5% charcoal dextran-treated FCS (CDFCS) for 2 days, after which the cells were maintained in improved MEM (IMEM) minus phenol red plus 5% CDFCS for 6 days prior to use in experiments. All media included penicillin (100 units/ml), streptomycin (100 μg/ml), and gentamycin (25 μg/ml) (GIBCO). Long-term TOT-maintained MCF-7 cells were derived from these parental MCF-7 cells by growth for over 6 months in the presence of 10−6 M TOT (13).
MDA-MB-231 human breast cancer cells were maintained in Leibovitz’s L15 medium with 10 mM Hepes, 5% calf serum, 100 units penicillin/ml, 100 μg streptomycin/ml, 25 μg gentamycin/ml, 6 ng bovine insulin/ml, 3.75 ng hydrocortisone/ml, and 16 μg glutathione/ml. Cells were then grown in MEM plus phenol red supplemented with 5% charcoal dextran-treated calf serum (CDCS) for 2 days prior to use in experiments.
Cells used in all experiments were in the log phase of growth and were at 30–50% of confluence.
Isolation of RNA.
Total RNA was isolated using the RNA extraction kit from Pharmacia.
Differential Display.
Differential display was performed using the RNAimage kit from GenHunter (Nashville, TN). DNA-free RNA was obtained by treatment of total RNA with DNase I (Promega) in the presence of placental RNase inhibitor (GIBCO) for 30 min at 37°C. After phenol/chloroform extraction and ethanol precipitation, three reverse transcription reactions were performed for each RNA sample using 0.2 μg of DNA-free total RNA in 1× reverse transcription buffer (25 mM Tris·Cl, pH 8.3/37.6 mM KCl/1.5 mM MgCl2/5 mM DTT) and 20 μM each of dATP, dCTP, dGTP, and dTTP, and 0.2 μM of HindIII restriction site-containing one-base anchored oligo(dT) primers [either H-T11A (5′-AAGCTTTTTTTTTTTA-3′), H-T11C (5′-AAGCTTTTTTTTTTTC-3′), or HT11G (5′-AAGCTTTTTTTTTTTG-3′)]. After the solution was heated at 65°C for 5 min and cooled to 37°C, 100 units of Moloney murine leukemia virus reverse transcriptase was added for 1 hr. PCRs were performed in reaction mixtures containing 0.1 vol of reverse transcription reaction mixture, 1× PCR buffer (10 mM Tris·Cl, pH 8.4/50 mM KCl/1.5 mM MgCl2/0.001% gelatin), 2 μM each of dATP, dCTP, dGTP, and dTTP, 0.2 μM of HindIII restriction site-containing arbitrary 13-mer oligonucleotide [either H-AP1 (5′-AAGCTTGATTGCC-3′), H-AP2 (5′-AAGCTTCGACTGT-3′), H-AP3 (5′-AAGCTTTGGTCAG-3′), H-AP4 (5′-AAGCTTCTCAACG) or H-AP5 (5′-AAGCTTAGTAGGC-3′)], 0.2 μM of the corresponding HindIII restriction site-containing one-base anchored oligo(dT) primer, 0.1 μCi of 35S-dATP (1 Ci = 37 GBq), and 1 unit of AmpliTaq DNA polymerase (Perkin–Elmer/Cetus). Light mineral oil was overlaid and the PCR reactions were performed at 94°C for 30 sec and 40 cycles of 94°C for 30 sec, 40°C for 2 min, 72°C for 30 sec, followed by 72°C for 5 min. Stop buffer (95% formamide/10 mM EDTA, pH 8.0/0.09% xylene cyanol/0.09% bromophenol blue) was added to each sample and heated at 80°C for 2 min before loading on 6% polyacrylamide sequencing gels. After electrophoresis the gels were exposed to Kodak XAR-5 film for 48 hr. Any band differentially expressed in parental vs. long-term TOT-maintained cells was identified and the PCR was repeated to confirm the findings.
Any cDNA species that was differentially expressed was recovered from the dried DNA sequencing gel (21) and reamplified using the same primer set and PCR conditions as used in the mRNA display, except that the dNTP concentrations were at 20 μM instead of 2 μM and no isotope was added. Reamplified cDNA was run on a 1.2% agarose gel and purified using the QIAEX kit from Qiagen (Chatsworth, CA). Reamplified cDNA was subsequently cloned into the PCRII vector using the TA cloning system from Invitrogen and sequenced using the Sequenase kit (United States Biochemical).
Northern Blot Analysis.
Gel-purified reamplified cDNA was random-primer labeled using the Ready-to-Go DNA labeling kit from Pharmacia for Northern blot analysis to verify differential expression. Twenty micrograms of total RNA was separated by electrophoresis, transferred to nitrocellulose support, and hybridized with random-primer-labeled cDNA (22).
Transfections.
MDA-MB-231 cells were transfected as described (23). Cells were seeded for transfection at 7.5 × 105 per 60-mm dish in IMEM minus phenol red containing 5% CDCS. Cells were transfected by the CaPO4 coprecipitation method 24 hr later with 3 μg of the GstYa-GH reporter constructs, 60 ng of ER expression vector, 100 ng of pCMVβ β-gal internal control plasmid, and 4.84 μg of pTZ19 carrier DNA. Cells remained in contact with the precipitate for 5 hr and were then subjected to a 2-min glycerol shock (20% in IMEM minus phenol red plus 5% CDCS). Cells were rinsed with Hanks’ balanced salt solution and given fresh media with or without hormones.
Media were collected 48 hr after hormone treatment for GH (reporter gene) assay. GH production was measured using the GH transient gene expression assay from Nichols Institute (San Juan Capistrano, CA). Cells were harvested and processed to measure β-gal activity, to normalize for transfection efficiency, as described (24).
QR Assay.
MCF-7 cells were plated at 2.5 × 105 cells per 100-mm plate in IMEM minus phenol red containing 5% CDFCS. Hormone treatments were started the day after plating. For extended treatment regimens, cells were fed with fresh media containing a hormone(s) every 2 days. Before harvesting, the cell monolayer was washed twice with ice-cold phosphate-buffered saline without calcium and magnesium after removal of media. The cells were scraped from the plates and collected by centrifugation (800 × g for 5 min). The cell pellet from each flask was homogenized briefly in 50 mM phosphate buffer (pH 7.5). The homogenate was centrifuged at 14,000 rpm for 15 min at 4°C and the supernatant was removed and frozen at −80°C until assayed for QR activity.
To assay for QR enzyme activity by the spectrophotometric method, menadione was used as a substrate and cytochrome c as the terminal electron acceptor (25). After the mixing of 10 μM menadione, 70 μM cytochrome c, and cytosol in pH 7.5 buffer, the tube contents were transferred to a cuvette and the baseline A550 was determined in a spectrophotometer. Following addition of NADPH (final concentration, 0.5 mM) and rapid mixing of the contents, the linear change in absorbance at 550 nm was recorded at 30-sec periods. The enzyme activity was calculated as nanomoles of cytrochrome c reduced per min and the specific QR activity is expressed as units (nanomoles of cytochrome c reduced per min) per mg of protein.
RESULTS
Induction of QR Activity in MCF-7 Cells by Antiestrogens.
Through the technique of differential RNA display, we observed QR to be a species that was present at a much higher level in an MCF-7 breast cancer cell subline that had been grown long term (over 6 months) in our laboratory in the presence of high (10−6 M) levels of the antiestrogen TOT, as compared with the parental MCF-7 cells grown in the absence of TOT (Fig. 1). The nucleotide sequence of the differentially displayed cDNA species showed 96% homology with exon 6 of the gene for human NAD(P)H:quinone oxidoreductase. As shown in the Northern blot in Fig. 1 using this cDNA as a probe, we found that QR RNA was present at 8 times greater abundance in the long-term TOT-maintained cells than in the parental MCF-7 cells. That the differentially expressed message is the transcription product of the QR gene is supported by the 4-fold higher QR enzyme activity in the long-term tamoxifen-maintained cell line (data not shown). We therefore examined the ability of antiestrogen to evoke increases in QR activity over time when parental MCF-7 cells were treated with antiestrogens.
Figure 1.
MCF-7 breast cancer cells grown long term in the presence of TOT express elevated levels of QR RNA. (A) Differential display gel showing cDNA species from parental and long-term TOT-maintained MCF-7 cells. Differential display was performed as described. RNA samples from parental and long-term TOT-maintained MCF-7 cells were compared by differential display using a one-base anchored primer, H-T11G (5′-AAGCTTTTTTTTTTTG-3′), in conjunction with a 13-mer arbitrary primer, H-AP1 (5′-AAGCTTGATTGCC-3′). QR partial cDNA (indicated by arrow) was expressed at a much higher level in long-term TOT-maintained MCF-7 cells and was subsequently recovered, reamplified, and gel-purified as described. (B) Northern blot analysis to verify differential expression of QR RNA. Total RNA was collected from MCF-7 parental cells and cells grown for over 6 months in 10−6 M TOT. Equal amounts (20 μg) of total RNA were separated by electrophoresis as described. The blot was probed with the random-primer-labeled QR partial cDNA. As an RNA loading control the same blot was reprobed with 36B4 cDNA.
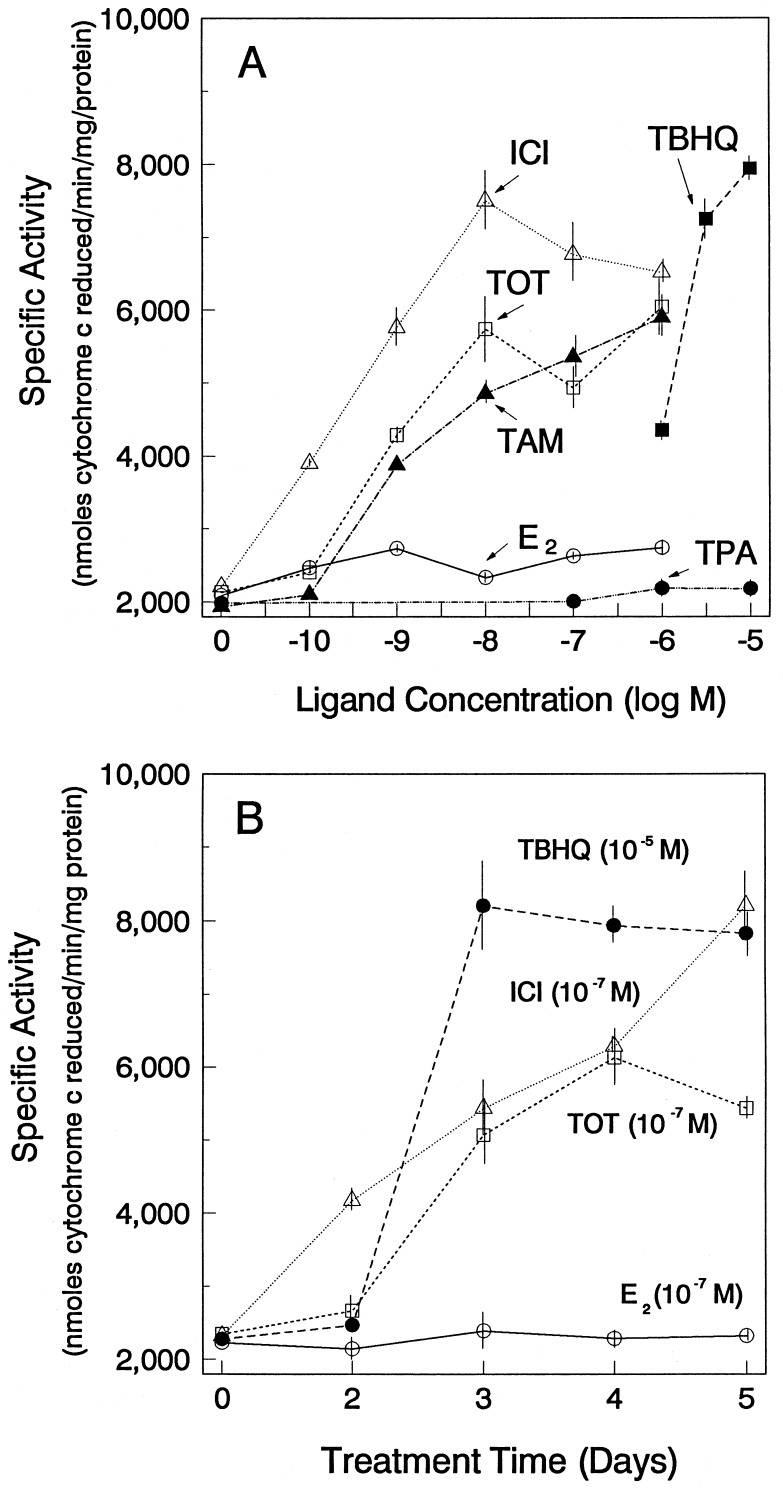
As shown in Fig. 2, a 3- to 4-fold increase in QR activity was observed upon treatment with the antiestrogens TOT, tamoxifen, and ICI. The induction of QR activity by antiestrogens occurred in a dose-dependent manner (Fig. 2A), and maximal stimulation was obtained with a relatively low concentration of the antiestrogens (10−8 M for TOT and ICI, ≈10−7 M for tamoxifen). The concentrations of antiestrogen required for stimulation of QR activity were substantially lower than those needed for stimulation by a previously identified potent inducer of QR activity in other systems, TBHQ. Maximal induction of QR activity occurred after 3–4 days of treatment with the antiestrogens (Fig. 2B), and the time course and magnitude of induction, was similar to that achieved by TBHQ. Of note, no increase in QR activity was observed in the presence of E2 (Fig. 2) or the tumor promoter phorbol ester TPA (Fig. 2A). However, as shown in Fig. 3, the antiestrogen-induced increase in QR activity was fully inhibited by E2. These observations suggest that the modulation of QR activity by antiestrogens is mediated by the ER. In contrast, E2 did not affect the induction of QR by TBHQ (Fig. 3).
Figure 2.
Antiestrogens, but not estrogen, induce NAD(P)H:quinone oxidoreductase enzyme activity in MCF-7 breast cancer cells: Concentration-dependence and time course. (A) Effect of different compounds on QR activity in MCF-7 cells. Cells were treated with different concentrations of E2, TOT, tamoxifen (TAM), ICI, TBHQ, or TPA. Cells were harvested after 4 days of treatment. (B) Time course of induction of QR activity in MCF-7 breast cancer cells. MCF-7 cells were treated with the indicated concentrations of E2, TOT, ICI, or TBHQ. Cells were then harvested at the indicated time points. Cytosolic extracts were assayed for QR activity as described. Values are the means ± SE from three separate experiments.
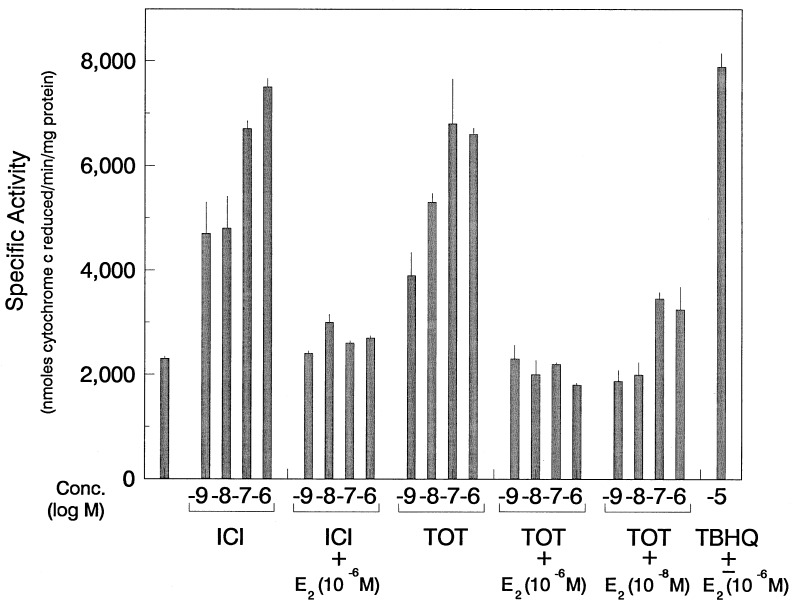
Figure 3.
E2 inhibits antiestrogen-mediated induction of NAD(P)H:quinone oxidoreductase enzyme activity in MCF-7 cells. MCF-7 cells were treated with different concentrations of TOT and ICI or with 10−5 M TBHQ with or without E2 at the concentrations indicated. Cells were harvested after 4 days of treatment. Cytosolic extracts were assayed for QR activity as described. A similar extent of inhibition of QR activity by TOT and ICI was observed with 10−6 M and 10−7 M E2, and therefore only 10−6 M and 10−8 M E2 data are shown. Values are the means ± SE from three separate experiments.
Induction of EpRE Enhancer Activity in 231 Breast Cancer Cells.
To examine more definitively the role of the ER in the antiestrogen-mediated induction, an ER-negative breast cancer cell line, MDA-MB-231, was used. Since regulation of the QR gene, as well as other genes involved in detoxification such as the GST Ya gene, is known to be mediated through ARE or EpRE (14, 15), we used reporter constructs containing the EpRE enhancer region. Cells were transfected with a p41–284GstYa-GH reporter construct (with the 41-bp EpRE-containing 5′ upstream region from the murine GST Ya gene and its homologous promoter fused to the human growth hormone structural sequences) in the absence or presence of an expression vector for the wild-type ER or for mutant forms of the ER lacking DNA binding ability (ERDBDmut) or transcriptional activity (ERS554fs).
A significant increase in transcriptional activity of the EpRE-containing reporter construct in the presence of TOT was observed in 231 cells only when cells were cotransfected with an expression vector for the wild-type ER (Fig. 4). Cells transfected with the empty control pCMV vector (Fig. 4 Right), lacking the ER cDNA, did not show an increase in GH production in response to antiestrogen. Maximal induction of GH production by antiestrogens was comparable to that evoked by TBHQ. Of note, however, TBHQ stimulation of the reporter gene construct did not require the ER and was equally robust in the presence or absence of ER. Consistent with the lack of any significant increase in QR enzyme activity in the presence of E2 in MCF-7 cells (Fig. 2), no increase in the transcriptional activity of EpRE-containing constructs was observed with E2. Rather, TOT-mediated induction of EpRE-reporter transcriptional activity was repressed in the presence of E2, consistent with an important role for the ER in the antiestrogen-mediated activation. E2, by presumably binding to the ER in a manner competitive with antiestrogen, inhibited the TOT-mediated increase in transcriptional activity. Antiestrogen-mediated transcriptional activation was not observed with an ER DNA binding mutant, which lacks most (amino acids 185–251) of the ER DNA binding domain and therefore does not bind to DNA, suggesting the requirement for an interaction of the ER either directly with DNA elements within the 41-bp EpRE-containing region or with an ERE-containing gene, whose stimulation is required for production of/or regulation of a protein factor that directly binds to the EpRE-containing enhancer region and is responsible for the antiestrogen stimulation. Likewise, a transcriptionally inactive ER (S554fs), which binds antiestrogen but lacks activation function-2 activity (19), failed to stimulate EpRE reporter gene transactivation providing further evidence that functional ER is required (Fig. 4).
Figure 4.
Antiestrogen induction of EpRE-GstYa-GH reporter gene activity in 231 breast cancer cells is mediated by the ER. 231 cells were transfected with the p41–284GstYa-GH reporter construct along with an expression vector for the wild-type human ER, an expression vector for a DNA binding mutant of the ER (ERDBDmut, missing amino acids 185–251), an expression vector for a mutant ER that lacks activation function-2 activity (ERS554fs), or the empty expression vector missing the ER cDNA (pCMV). The cells were also transfected with a β-gal internal control reporter to correct for transfection efficiency. Cells were then treated for 48 hr with varying concentrations of TOT, E2, or TBHQ as indicated. Media were collected for measurement of GH levels as described. The cells were then harvested for measurement of β-gal activity. Values are the means ± SE from three separate experiments.
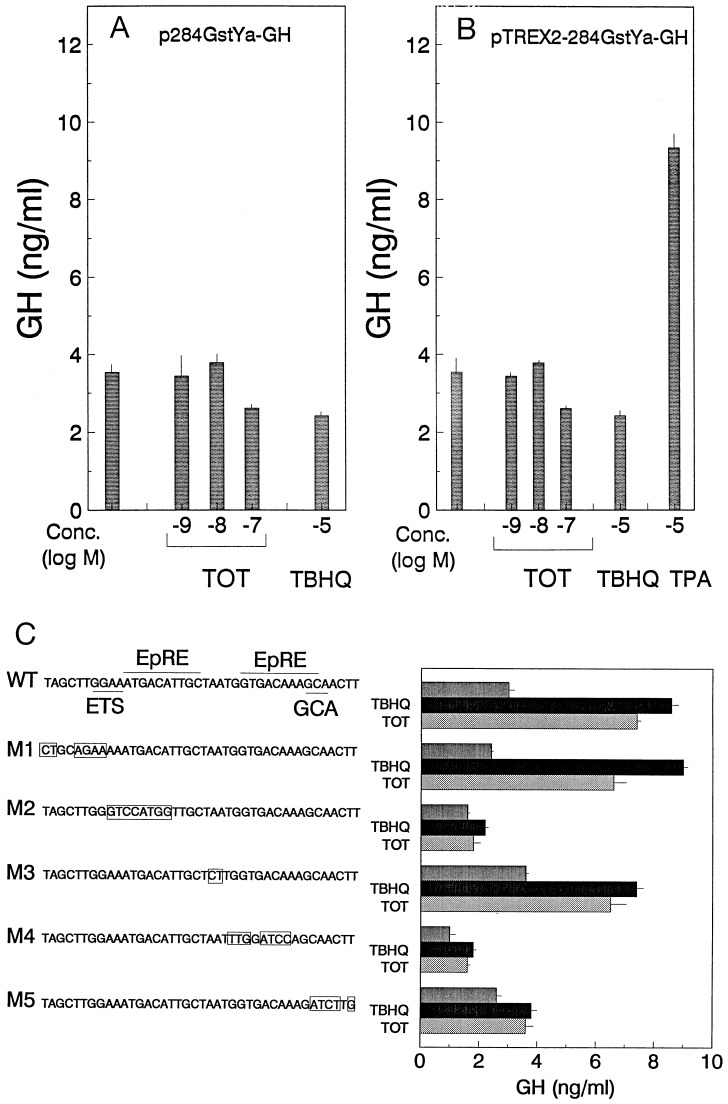
The absence of stimulation by antiestrogen of a reporter construct lacking the 41-bp EpRE-containing fragment indicates that TOT stimulation of Gst Ya transcriptional activity requires this region (Fig. 5A). Because the EpRE/ARE is very similar to a TRE, and TOT can have regulatory effects on transcription through TRE sites in some cells (26), we also examined whether TOT treatment could elicit an increase in transcriptional activity through a TRE site. While TPA was able to stimulate transcriptional activity from the TRE-containing reporter construct, no increase in transcriptional activity was elicited by TOT or TBHQ (Fig. 5B). Thus, TOT-mediated induction of QR activity is unlikely to be attributable to endogenous AP1 cell activity.
Figure 5.
Antiestrogen induction of GstYa-GH reporter activity is mediated by an EpRE. 231 cells were transfected with an expression vector for the ER along with (A) pGstYa-GH reporter construct (missing the 41-bp EpRE-containing fragment), (B) TREX2-GstYa-GH reporter construct, or (C) mutated p41–284GstYa-GH reporter constructs. The nucleotide sequence of the 41-bp EpRE-containing fragment. The consensus EpRE, ETS binding site, and GCA box are also indicated. The mutated nucleotides are indicated by the boxed regions in mutants 1–5. The cells were also transfected with the β-gal internal reporter to correct for transfection efficiency. They were then treated with TOT (10−7 M) or TBHQ (10−5 M) for 48 hr. Media were collected for measurement of GH levels and the cells were then harvested for measurement of β-gal activity as described. Values are the means ± SE from three separate experiments.
To document that stimulation by the antiestrogen-occupied ER observed in Fig. 4 was mediated through the EpRE within the Gst Ya 41-bp region, we did oligonucleotide mutagenesis over this region (Fig. 5C). Introduction of specific base pair changes at the EpREs (M2 and M4) eliminated the response to both TBHQ and TOT. Mutation of the GCA box (M5), a sequence that is highly conserved among EpRE-containing fragments of different genes but is of unknown function (reviewed in ref. 27), greatly decreased both TBHQ and TOT-induced transcriptional activity. Mutations at other sites within the 41-bp fragment (M1 and M3) had little effect on the response to TBHQ or TOT. These findings, shown in Fig. 5C, indicate that loss of stimulation by antiestrogen or by TBHQ map identically to the EpRE element.
DISCUSSION
We have shown that QR, a phase 2 detoxification enzyme, is markedly induced by antiestrogens in breast cancer cells. Moreover, the antiestrogens stimulated reporter gene transcription from EpRE/ARE-containing constructs, and mutagenesis and other studies showed that the stimulation by antiestrogen and by TBHQ mapped identically to the 10-bp EpRE element. The transcriptional activation by antiestrogens appears to be mediated by the ER and requires a DNA-binding, transcriptionally active ER. Thus, antiestrogens may function as anticancer agents not merely by inhibiting estrogen-mediated activation of gene transcription, but also by being able to activate a separate subset of genes, which may have beneficial effects.
Our experiments indicate that QR can be activated by ER-independent and ER-dependent pathways. Activation by antiestrogens depends on the presence of a functional ER and requires much lower doses of compound (10−10–10−8 M) than stimulation by known activators of QR such as TBHQ (10−6–10−5 M). While the signals between these two pathways may be integrated at some level, the ER-dependent pathway—which may involve induction or regulation of some protein that regulates the EpRE—must be different in part from the electrophile-dependent pathway used by TBHQ, where the ER is not at all required for QR stimulation. Electrophilic metabolites are known to be generated from tamoxifen and E2; however, it is not clear that they would be generated from TOT, ICI164,384, or tamoxifen more so than from E2, which reverses the activation, nor are they likely to be produced in sufficient quantities from the low concentrations of these antiestrogens used in our experiments. Therefore, we think it is unlikely that the generation of electrophilic metabolites can account for the ER-dependent activity of the antiestrogens.
The EpRE-binding proteins that mediate induction of phase 2 genes by phenolic antioxidants have not been identified, but they do not appear to be AP1 (Fos/Jun) proteins (28–30). Likewise, regulation through the EpRE by antiestrogens that we have observed in these studies does not appear to be due to endogenous AP1 activity. Our analyses indicate that although the ER is required for antiestrogen-mediated induction of transcriptional activity of EpRE-containing genes, the ER is not required for TBHQ-mediated activation. Furthermore, the 41-bp EpRE-containing fragment shares no homology with an estrogen response element. The time course of endogenous QR enzyme activation by the antiestrogens and by TBHQ (Fig. 2), as well as the time course for QR mRNA increases (by 24 hr) and for stimulation of EpRE-reporter gene activity by both agents (increases first observed at 36 hr, data not shown), suggest that these may be secondary, rather than primary, gene transcriptional effects. Thus, the antiestrogen–ER complex may enhance the production of (or activity of) stimulatory factors that bind to the EpRE or promote the release of inhibitory factors. Further studies will be needed to completely elucidate the mechanisms of antiestrogen regulation.
The QR gene is unusual in that it is an antiestrogen-stimulated gene and shows “reversed pharmacology,” being induced in ER-containing cells by antiestrogen treatment and suppressed when the ER is occupied with estrogen. Indeed, very few genes have been found to be up-regulated by antiestrogens, transforming growth factor β3 (31) and an uncharacterized secreted protein of ≈39 kDa (32, 33) being the only two identified to date.
Increased QR activity in the presence of antiestrogens may have several important consequences. Although regulation of the effectiveness of cancer chemotherapeutic agents is complex, antiestrogen treatment might enhance the sensitivity of cells to those agents that are activated by quinone reduction, such as mitomycin C and aziridylbenzoquinones (34). Also, being a two-electron reductant, QR can also provide protection against the deleterious effects of reactive oxygen species (35). This may contribute to the beneficial effects of antiestrogens in cancer therapy and in chemoprevention. In addition, the potential for antiestrogens to modulate the activity of EpRE-containing genes has broad implications regarding the regulation of many phase 2 detoxification enzymes and other genes whose transcription is under the control of EpRE/AREs.
Acknowledgments
We thank Paul Talalay (The Johns Hopkins University School of Medicine) and Pierre Chambon (Institut National de la Santé et de la Recherche Médicale, Illkirch, France) for providing plasmids and John Katzenellenbogen (University of Illinois) for helpful discussions. This work was supported by National Institutes of Health Grant CA18119 and U.S. Army Breast Cancer Program Grant DAMD17-94-J-4205 (B.S.K.) and by a postdoctoral fellowship from the Susan G. Komen Breast Cancer Foundation (M.M.M.).
ABBREVIATIONS
- ER
estrogen receptor
- E2
estradiol
- QR
quinone reductase
- TBHQ
tert-butylhydroquinone
- EpRE/ARE
electrophile/antioxidant response element
- GST Ya
glutathione S-transferase Ya subunit
- TOT
trans-hydroxytamoxifen
- GH
growth hormone
- TPA
12-tetradecanoate 13-acetate
- TRE
TPA response element
- β-gal
β-galactosidase
- ICI
ICI182,780
References
- 1.Katzenellenbogen B S. J Natl Cancer Inst. 1991;83:1434–1435. doi: 10.1093/jnci/83.20.1434. [DOI] [PubMed] [Google Scholar]
- 2.Read L D, Katzenellenbogen B S. In: Genes, Oncogenes, and Hormones: Advances in Cellular and Molecular Biology of Breast Cancer. Dickson R B, Lippman M E, editors. Boston: Kluwer; 1991. pp. 277–299. [Google Scholar]
- 3.Wakeling A E. Biochem Pharmacol. 1995;49:1545–1549. doi: 10.1016/0006-2952(94)00528-t. [DOI] [PubMed] [Google Scholar]
- 4.Osborne C K, Elledge R M, Fuqua S A W. Sci Am Sci Med. 1996;3:32–41. [Google Scholar]
- 5.McDonald C C, Stewart H J. Br Med J. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Love R R, Richard M D, Mazess R B, Barden H S, Epstein S, Newcomb P A, Jordan V C, Carbone P P, DeMets D L. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 7.Bhimani R S, Troll W, Grunberger D, Frenkel K. Cancer Res. 1993;53:4528–4533. [PubMed] [Google Scholar]
- 8.Ahotupa M, Hirsimaki P, Parssinen R, Mantyla E. Carcinogenesis. 1994;15:863–868. doi: 10.1093/carcin/15.5.863. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman H. Trends Pharmacol Sci. 1994;15:83–88. doi: 10.1016/0165-6147(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 10.Talalay P. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 11.Prestera T, Zhang Y, Spencer S R, Wilczak C A, Talalay P. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 12.Liang P, Pardee A B. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- 13.Herman M E, Katzenellenbogen B S. J Steroid Biochem Mol Biol. 1996;59:121–134. doi: 10.1016/s0960-0760(96)00114-8. [DOI] [PubMed] [Google Scholar]
- 14.Friling R S, Bensimon A, Tichauer Y, Daniel V. Proc Natl Acad Sci USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushmore T H, Morton M R, Pickett C B. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 16.Prestera T, Talalay P. Proc Natl Acad Sci USA. 1995;92:8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel T A, Roberts J D, Zakour R A. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 18.Mead D, Szczesna-Skorupa E, Kemper B. Protein Eng. 1986;1:67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Wrenn C K, Katzenellenbogen B S. J Biol Chem. 1993;268:24089–24098. [PubMed] [Google Scholar]
- 20.Kumar V, Green S, Staub A, Chambon P. EMBO J. 1986;5:2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang P, Averboukh L, Pardee A B. Nucleic Acids Res. 1993;21:3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H, Ng P A, Katzenellenbogen B S. Mol Endocrinol. 1991;5:1323–1330. doi: 10.1210/mend-5-9-1323. [DOI] [PubMed] [Google Scholar]
- 23.Montano M M, Müller V, Trobaugh A, Katzenellenbogen B S. Mol Endocrinol. 1995;9:814–825. doi: 10.1210/mend.9.7.7476965. [DOI] [PubMed] [Google Scholar]
- 24.Reese J C, Katzenellenbogen B S. J Biol Chem. 1991;266:10880–10887. [PubMed] [Google Scholar]
- 25.Jaiswal A K, McBride O W, Adesnik M, Nebert D W. J Biol Chem. 1988;263:13572–13578. [PubMed] [Google Scholar]
- 26.Webb P, Lopez G N, Uht R M, Kushner P J. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 27.Jaiswal A K. Biochem Pharmacol. 1994;48:439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen T, Rushmore T H, Pickett C B. J Biol Chem. 1994;269:13656–13662. [PubMed] [Google Scholar]
- 29.Wang B, Williamson G. Biochim Biophys Acta. 1994;1219:645–652. doi: 10.1016/0167-4781(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 30.Yoshioka K, Deng T, Cavigelli M, Karin M. Proc Natl Acad Sci USA. 1995;92:4972–4976. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang N N, Venugopalan M, Hardikar S, Glasebrook A. Science. 1996;273:1222–1225. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]
- 32.Bronzert D, Silverman S, Lippman M. Cancer Res. 1987;47:1234–1238. [PubMed] [Google Scholar]
- 33.Sheen Y Y, Katzenellenbogen B S. Endocrinology. 1987;120:1140–1151. doi: 10.1210/endo-120-3-1140. [DOI] [PubMed] [Google Scholar]
- 34.Ross D, Siegel D, Beall H, Prakash A S, Mulcahy R T, Gibson N W. Cancer Metastasis Rev. 1993;12:83–101. doi: 10.1007/BF00689803. [DOI] [PubMed] [Google Scholar]
- 35.Cadenas E. In: Oxidative Stress and Antioxidant Defenses in Biology. Ahmad S, editor. New York: Chapman and Hall; 1995. pp. 1–61. [Google Scholar]