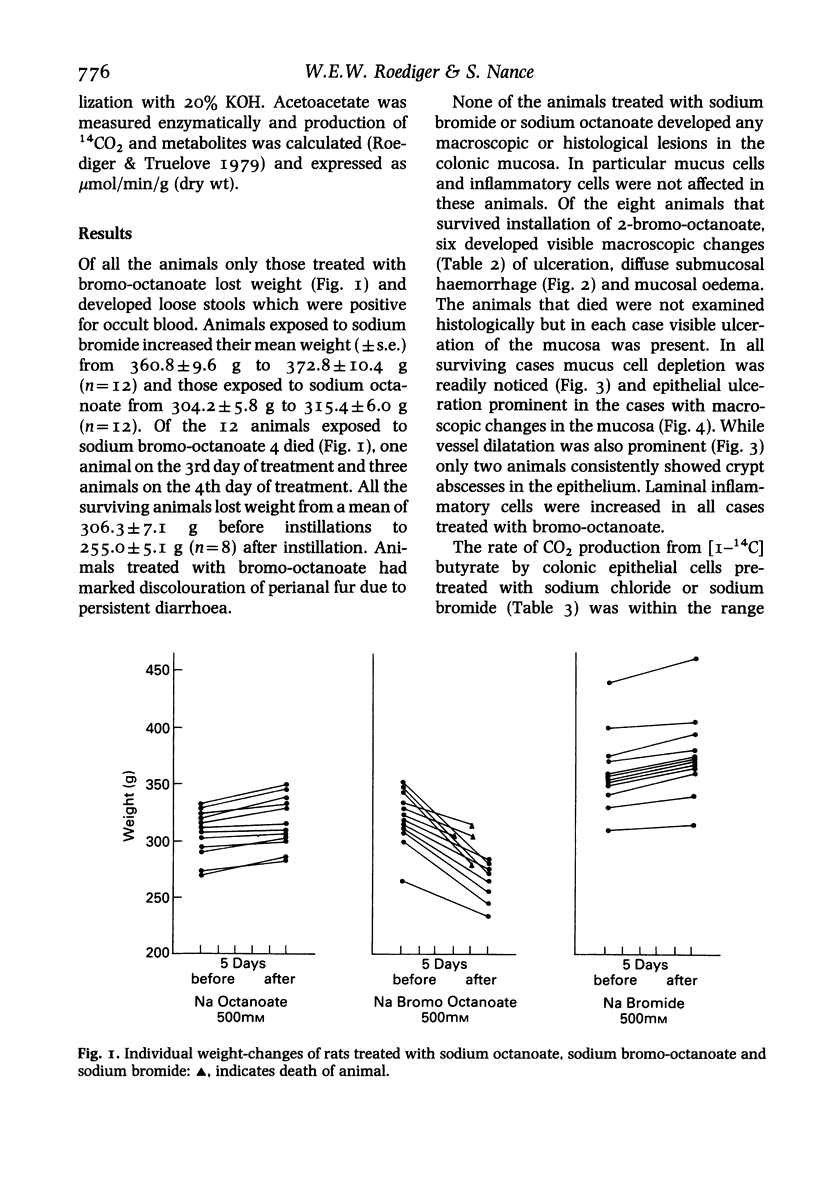
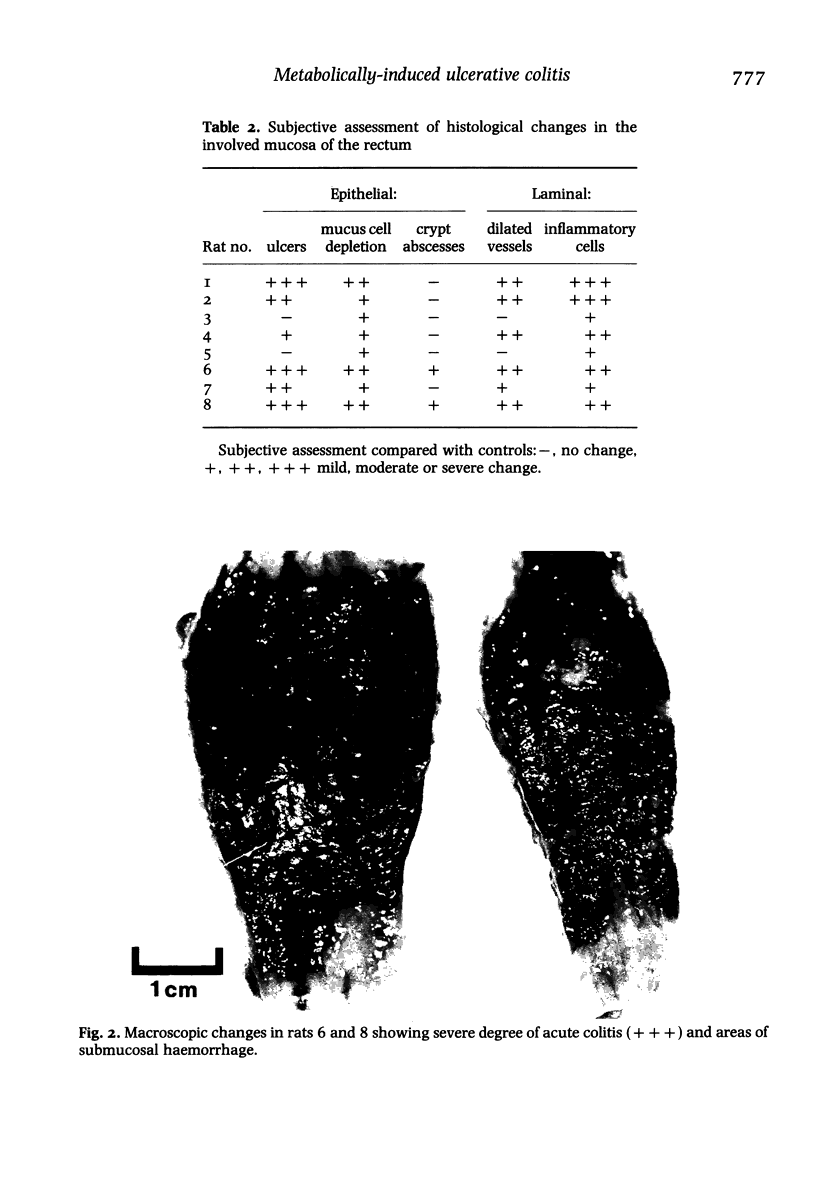
Abstract
There is some evidence that failure of fatty acid or beta-oxidation in the epithelium of the colonic mucosa is associated with the development of ulcerative colitis. We tested the hypothesis that inhibition of fatty acid oxidation in the colonic mucosa of the rat reproduces the histological, clinical and biochemical lesions of acute ulcerative colitis of man. A specific inhibitor of beta-oxidation, sodium 2-bromo-octanoate, was instilled rectally for 5 days or exposed to isolated colonic epithelial cells which were subsequently tested for their ability to beta-oxidize n-butyrate. Weight loss, bloody diarrhoea and histological lesions occurred with 2-bromo-octanoate treated rats but not control animals. Ketogenesis and 14CO2 production was inhibited by 2-bromo-octanoate. Of 12 animals mucosal ulceration developed in six out of eight surviving animals and in all four animals that died. Ulceration, mucus cell depletion, vessel dilatation and increases of inflammatory cells were the most prominent histological changes. Present observations indicate that inhibition of beta-oxidation produces acute colitis and suggests that inhibition of beta-oxidation is primary rather than secondary in the genesis of ulcerative colitis. A search for agents producing such biochemical lesions in man should be undertaken.
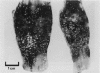
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S., Newsholme E. A. Fuel utilization in colonocytes of the rat. Biochem J. 1985 Nov 1;231(3):713–719. doi: 10.1042/bj2310713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enser M. Fatty acids and intestinal metabolism. Biochem J. 1964 Nov;93(2):290–297. doi: 10.1042/bj0930290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S. J., Hird F. J. Ketogenesis from butyrate and acetate by the caecum and the colon of rabbits. Biochem J. 1972 Dec;130(3):785–790. doi: 10.1042/bj1300785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsner J. B., Shorter R. G. Recent developments in "nonspecific" inflammatory bowel disease (first of two parts). N Engl J Med. 1982 Apr 1;306(13):775–785. doi: 10.1056/NEJM198204013061304. [DOI] [PubMed] [Google Scholar]
- MacPherson B., Pfeiffer C. J. Experimental colitis. Digestion. 1976;14(5-6):424–452. doi: 10.1159/000197966. [DOI] [PubMed] [Google Scholar]
- Marty J., Vernay M. Absorption and metabolism of the volatile fatty acids in the hind-gut of the rabbit. Br J Nutr. 1984 Mar;51(2):265–277. doi: 10.1079/bjn19840031. [DOI] [PubMed] [Google Scholar]
- Nelson R. A. Intestinal transport, coenzyme A, and colitis in pantothenic acid deficiency. Am J Clin Nutr. 1968 May;21(5):495–501. doi: 10.1093/ajcn/21.5.495. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Sherratt H. S. Inhibitors of beta-oxidation. Biochem Soc Trans. 1978;6(1):84–88. doi: 10.1042/bst0060084. [DOI] [PubMed] [Google Scholar]
- Peters R. A. The biochemical lesion and its historical development. Br Med Bull. 1969 Sep;25(3):223–226. doi: 10.1093/oxfordjournals.bmb.a070707. [DOI] [PubMed] [Google Scholar]
- Raaka B. M., Lowenstein J. M. Inhibition of fatty acid oxidation by 2-bromooctanoate. Including effects of bromooctanoate on ketogenesis and gluconeogenesis. J Biol Chem. 1979 May 10;254(9):3303–3310. [PubMed] [Google Scholar]
- Rabin B. S., Rogers S. J. A cell-mediated immune model of inflammatory bowel disease in the rabbit. Gastroenterology. 1978 Jul;75(1):29–33. [PubMed] [Google Scholar]
- Roediger W. E., Deakin E. J., Radcliffe B. C., Nance S. Anion control of sodium absorption in the colon. Q J Exp Physiol. 1986 Apr;71(2):195–204. doi: 10.1113/expphysiol.1986.sp002978. [DOI] [PubMed] [Google Scholar]
- Roediger W. E., Lawson M. J., Kwok V., Grant A. K., Pannall P. R. Colonic bicarbonate output as a test of disease activity in ulcerative colitis. J Clin Pathol. 1984 Jun;37(6):704–707. doi: 10.1136/jcp.37.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E., Truelove S. C. Method of preparing isolated colonic epithelial cells (colonocytes) for metabolic studies. Gut. 1979 Jun;20(6):484–488. doi: 10.1136/gut.20.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T., Yajima T. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q J Exp Physiol. 1984 Jul;69(3):639–648. doi: 10.1113/expphysiol.1984.sp002850. [DOI] [PubMed] [Google Scholar]
- TRUELOVE S. C., ELLIS H., WEBSTER C. U. PLACE OF A DOUBLE-BARRELLED ILEOSTOMY IN ULCERATIVE COLITIS AND CROHN'S DISEASE OF THE COLON: A PRELIMINARY REPORT. Br Med J. 1965 Jan 16;1(5428):150–153. doi: 10.1136/bmj.1.5428.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Rostand S. G., Peterson M. J. Control factors affecting gluconeogenesis in perfused rat liver. Effects of 4-pentenoic acid. J Biol Chem. 1970 Jun;245(12):3242–3251. [PubMed] [Google Scholar]