Abstract
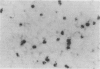
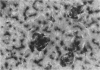
Hepatic silicosis was induced in rats by an intravenous injection of saline-suspended silica, 40 mg/kg of body weight. Changes in the liver were examined by biochemical, histological and histochemical methods. Infiltration of the liver parenchyma by polymorphonuclear leucocytes was observed only on the first day after silica treatment. Formation of silicotic nodules began on the first day by clustering of liver macrophages. A 22% increase in liver weight and a 67% increase in total liver DNA reflected accumulation of cells in the liver by day 28 after silica injection. Local cell division contributed to this increase. Almost all cells in the nodules contained carbon when the rats had been given ink before silica. Macrophages showed high activity of lysosomal esterases on the first few days after silica treatment; the activity disappeared later. Large granulomas containing hundreds of cells including lymphocytes were seen 226 days after treatment. Hydroxyproline content per gram of liver tissue increased by 35% and 58% by day 80 and 162, respectively. Connective tissue formed capsules around the nodules and grew to their inside. Activities of lysosomal enzymes, beta-D-galactosidase and acid proteases, in serum were increased by 20% and 300%, respectively, 35 days after treatment. Neither malondialdehyde concentration nor superoxide dismutase activity was elevated in silicotic liver.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bhatnagar R., Schirmer R., Ernst M., Decker K. Superoxide release by zymosan-stimulated rat Kupffer cells in vitro. Eur J Biochem. 1981 Sep;119(1):171–175. doi: 10.1111/j.1432-1033.1981.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Carmichael G. P., Jr, Targoff C., Pintar K., Lewin K. J. Hepatic silicosis. Am J Clin Pathol. 1980 May;73(5):720–722. doi: 10.1093/ajcp/73.5.720. [DOI] [PubMed] [Google Scholar]
- Comolli R. Cytotoxicity of silica and liberation of lysosomal enzymes. J Pathol Bacteriol. 1967 Jan;93(1):241–253. doi: 10.1002/path.1700930124. [DOI] [PubMed] [Google Scholar]
- Eide J., Gylseth B., Skaug V. Silicotic lesions of the bone marrow: histopathology and microanalysis. Histopathology. 1984 Jul;8(4):693–703. doi: 10.1111/j.1365-2559.1984.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Gömöri G. Silver Impregnation of Reticulum in Paraffin Sections. Am J Pathol. 1937 Nov;13(6):993–1002.5. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Silica stimulation of chemotactic factor release by guinea pig alveolar macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):381–390. [PubMed] [Google Scholar]
- STEGEMANN H. Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd. Hoppe Seylers Z Physiol Chem. 1958;311(1-3):41–45. [PubMed] [Google Scholar]
- Wolman M., Eldar T. Different patterns of macrophage activation induced by various agents. Cell Mol Biol Incl Cyto Enzymol. 1981;27(5):543–549. [PubMed] [Google Scholar]
- Zsoldos T., Tigyi A., Montskó T., Puppi A. Lipid peroxidation in the membrane damaging effect of silica-containing dust on rat lungs. Exp Pathol. 1983;23(2):73–77. doi: 10.1016/s0232-1513(83)80043-7. [DOI] [PubMed] [Google Scholar]