Abstract
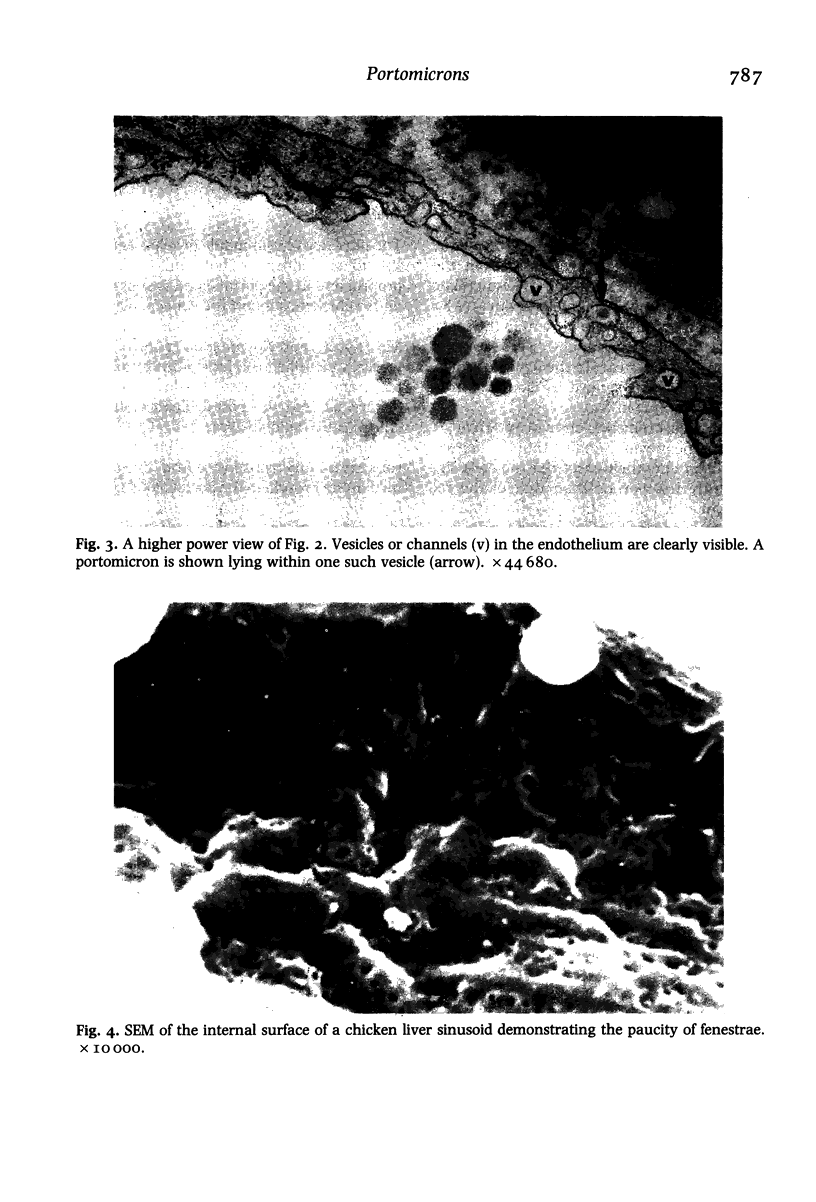
Dietary fat is transported in the chicken by portomicrons; these large lipoproteins enter the portal blood of the small intestinal villi. We have shown by electron microscopy that avian portomicrons resemble mammalian chylomicrons in size, but their mode of transport differs. Portomicrons enter the intestinal blood vessels through endothelial intracytoplasmic vesicles, whereas chylomicrons enter the intestinal lymphatics through gaps between endothelial cells. We have also shown that the sinusoidal endothelium of the chicken liver, like that of the mammal, is fenestrated. Because the fenestrae are relatively few in number, the endothelium is less porous in the chicken than in the rat. We postulate that this prevents the hepatocytes from being swamped by dietary fat, but makes the chicken susceptible to diet-induced atherosclerosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Rothfeld A. The form of absorption of lipids in the chicken, Gallus domesticus. Proc Soc Exp Biol Med. 1972 Dec;141(3):814–817. doi: 10.3181/00379727-141-36878. [DOI] [PubMed] [Google Scholar]
- Bundgaard M., Hagman P., Crone C. The three-dimensional organization of plasmalemmal vesicular profiles in the endothelium of rat heart capillaries. Microvasc Res. 1983 May;25(3):358–368. doi: 10.1016/0026-2862(83)90025-0. [DOI] [PubMed] [Google Scholar]
- CASLEY-SMITH J. R. The identification of chylomicra and lipoproteins in tissue sections and their passage into jejunal lacteals. J Cell Biol. 1962 Nov;15:259–277. doi: 10.1083/jcb.15.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Dzoga K., Fraser R., Wissler R. W. Stimulation of proliferation in stationary primary cultures of monkey and rabbit aortic smooth muscle cells. I. Effects of lipoprotein fractions of hyperlipemic serum and lymph. Exp Mol Pathol. 1976 Jun;24(3):346–359. doi: 10.1016/0014-4800(76)90070-8. [DOI] [PubMed] [Google Scholar]
- Florén C. H., Albers J. J., Bierman E. L. Uptake of chylomicron remnants causes cholesterol accumulation in cultured human arterial smooth muscle cells. Biochim Biophys Acta. 1981 Jan 26;663(1):336–349. doi: 10.1016/0005-2760(81)90219-8. [DOI] [PubMed] [Google Scholar]
- Florén C. H. Binding of apolipoprotein E-rich remnant lipoproteins to human liver membranes. Scand J Gastroenterol. 1984 Jun;19(4):473–479. [PubMed] [Google Scholar]
- Florén C. H. Hepatic binding of triglyceride-rich lipoproteins in humans. Scand J Clin Lab Invest. 1985 Oct;45(6):531–537. doi: 10.3109/00365518509155255. [DOI] [PubMed] [Google Scholar]
- Fraser R., Cliff W. J., Courtice F. C. The effect of dietary fat load on the size and composition of chylomicrons in thoracic duct lymph. Q J Exp Physiol Cogn Med Sci. 1968 Oct;53(4):390–398. doi: 10.1113/expphysiol.1968.sp001984. [DOI] [PubMed] [Google Scholar]
- Havel R. J. George Lyman Duff memorial lecture. Role of the liver in atherosclerosis. Arteriosclerosis. 1985 Nov-Dec;5(6):569–580. doi: 10.1161/01.atv.5.6.569. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Dobrilovic L. Aortic cholesterol and the plasma lipoproteins of the cholesterol-fed cockerel. Proc Soc Exp Biol Med. 1969 Jan;130(1):163–167. doi: 10.3181/00379727-130-33512. [DOI] [PubMed] [Google Scholar]
- Kim D. N., Geourzoung S. M., Schmee J., Lee K. T., Thomas W. A. Association of plasma intermediate density lipoproteins with atherogenic intimal proliferative activity in abdominal aortas of hyperlipidemic swine. Atherosclerosis. 1985 Dec;58(1-3):223–241. doi: 10.1016/0021-9150(85)90068-1. [DOI] [PubMed] [Google Scholar]
- PICK R., JAIN S., KAKITA C., JOHNSON P. EFFECT OF DEFATTED BRAIN EXTRACT AND SOY STEROLS ON PLASMA CHOLESTEROL LEVELS AND ATHEROGENESIS IN CHOLESTEROL-OIL FED COCKERELS. Proc Soc Exp Biol Med. 1965 Jul;119:850–854. doi: 10.3181/00379727-119-30318. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Wong H. Y., Newman H. A., Nightingale T. E., Frasinel C., Johnson F. B., Patel S., Coleman B. Effect of valium (diazepam) on experimental atherosclerosis in roosters. Artery. 1982;10(4):237–249. [PubMed] [Google Scholar]
- Sabesin S. M., Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977 Jul;18(4):496–511. [PubMed] [Google Scholar]
- Windler E., Havel R. J. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985 May;26(5):556–565. [PubMed] [Google Scholar]
- Wisse E., De Zanger R. B., Charels K., Van Der Smissen P., McCuskey R. S. The liver sieve: considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985 Jul-Aug;5(4):683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- Wright P. L., Smith K. F., Day W. A., Fraser R. Small liver fenestrae may explain the susceptibility of rabbits to atherosclerosis. Arteriosclerosis. 1983 Jul-Aug;3(4):344–348. doi: 10.1161/01.atv.3.4.344. [DOI] [PubMed] [Google Scholar]