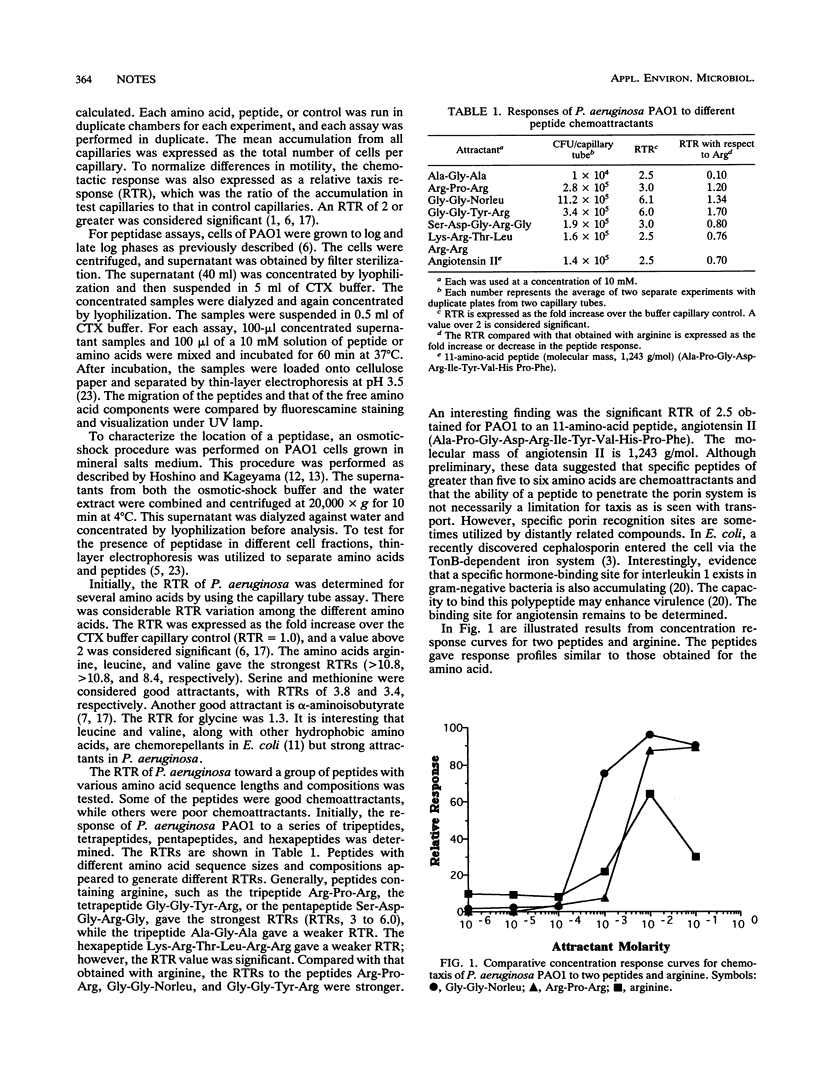
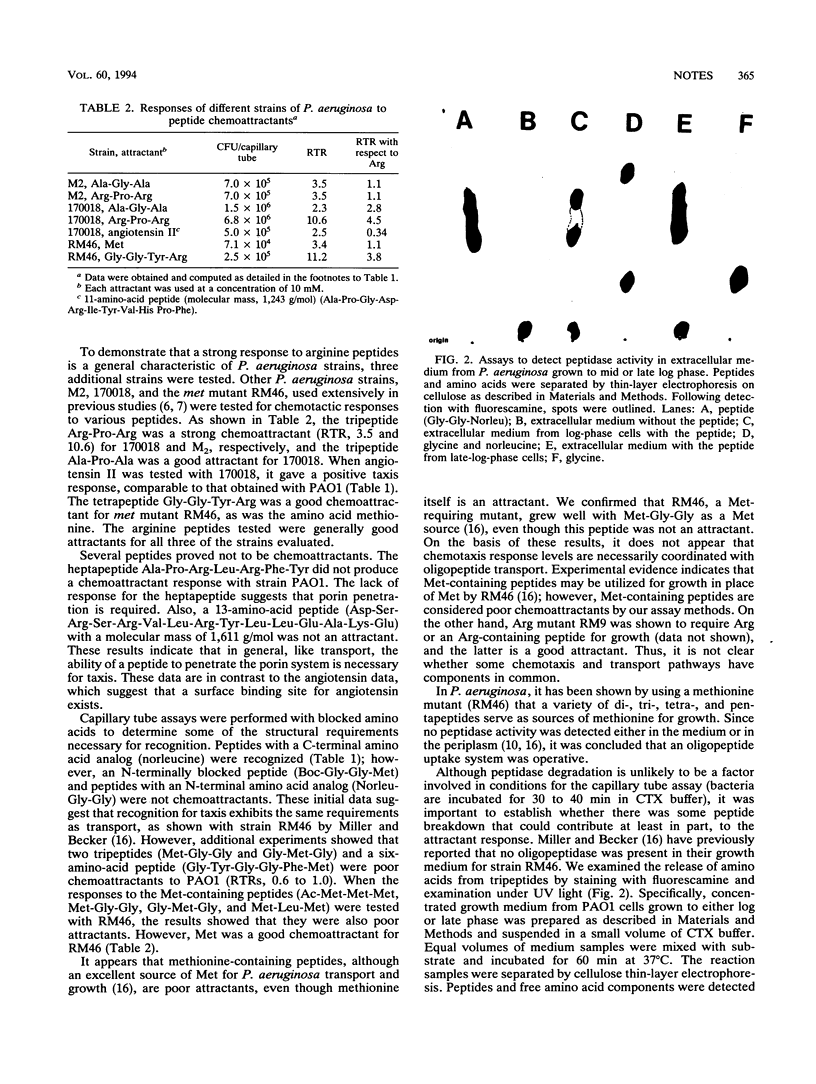
Abstract
A number of peptides were evaluated as chemoattractants for Pseudomonas aeruginosa. Several strains recognized tri-, tetra-, penta-, and hexapeptides in a capillary tube assay. Tripeptides altered at the carboxyl terminus were good attractants, whereas tripeptides altered at the amino terminus did not serve as chemoattractants. Methionine-containing peptides were relatively poor attractants. Arginine-containing peptides gave the best responses. Reduced responses to larger peptides suggest that porin penetration is required. No extracellular peptidase activity was detected. We conclude that oligopeptides are good attractants and that specificity for chemotactic recognition of oligopeptides exists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. Genetic analysis of Escherichia coli oligopeptide transport mutants. J Bacteriol. 1985 Feb;161(2):484–492. doi: 10.1128/jb.161.2.484-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. P. Behavioral responses in bacteria. Annu Rev Physiol. 1992;54:683–714. doi: 10.1146/annurev.ph.54.030192.003343. [DOI] [PubMed] [Google Scholar]
- Bergeron J., Macleod R. A., Dion P. Specificity of octopine uptake by Rhizobium and pseudomonas strains. Appl Environ Microbiol. 1990 May;56(5):1453–1458. doi: 10.1128/aem.56.5.1453-1458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Craven R. C., Montie T. C. Chemotaxis of Pseudomonas aeruginosa: involvement of methylation. J Bacteriol. 1983 May;154(2):780–786. doi: 10.1128/jb.154.2.780-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R., Montie T. C. Regulation of Pseudomonas aeruginosa chemotaxis by the nitrogen source. J Bacteriol. 1985 Nov;164(2):544–549. doi: 10.1128/jb.164.2.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán P. A., Amura C. R., García V. E., Cerquetti M. C., Sordelli D. O. Preliminary characterization of Pseudomonas aeruginosa peptide chemotactins for polymorphonuclear leukocytes. Infect Immun. 1992 Jun;60(6):2465–2469. doi: 10.1128/iai.60.6.2465-2469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M. P., Pearce S. R., Higgins C. F. Identification and localization of the membrane-associated, ATP-binding subunit of the oligopeptide permease of Salmonella typhimurium. Eur J Biochem. 1989 Mar 1;180(1):133–141. doi: 10.1111/j.1432-1033.1989.tb14623.x. [DOI] [PubMed] [Google Scholar]
- Haas M. W., Becker J. M., Miller R. V. Peptidase activity in the inner membrane of Pseudomonas aeruginosa. Biochim Biophys Acta. 1981 Apr 22;643(1):256–260. doi: 10.1016/0005-2736(81)90239-x. [DOI] [PubMed] [Google Scholar]
- Hedblom M. L., Adler J. Chemotactic response of Escherichia coli to chemically synthesized amino acids. J Bacteriol. 1983 Sep;155(3):1463–1466. doi: 10.1128/jb.155.3.1463-1466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Transport systems for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1979 Sep;139(3):705–712. doi: 10.1128/jb.139.3.705-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Wintenberg K., Anderson T., Montie T. C. Phosphorylated tyrosine in the flagellum filament protein of Pseudomonas aeruginosa. J Bacteriol. 1990 Sep;172(9):5135–5139. doi: 10.1128/jb.172.9.5135-5139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Blank V., Brade G., Higgins C. F. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature. 1986 May 15;321(6067):253–256. doi: 10.1038/321253a0. [DOI] [PubMed] [Google Scholar]
- Miller R. V., Becker J. M. Peptide utilization in Pseudomonas aeruginosa: evidence for membrane-associated peptidase. J Bacteriol. 1978 Jan;133(1):165–171. doi: 10.1128/jb.133.1.165-171.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naider F., Becker J. M. Multiplicity of oligopeptide transport systems in Escherichia coli. J Bacteriol. 1975 Jun;122(3):1208–1215. doi: 10.1128/jb.122.3.1208-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J. Chamber for bacterial chemotaxis experiments. Appl Environ Microbiol. 1976 Nov;32(5):729–730. doi: 10.1128/aem.32.5.729-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat R., Clark B. D., Wolff S. M., Dinarello C. A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991 Oct 18;254(5030):430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Studemeister A. E., DiVincenzo C. A., Lerner S. A. Resistance to imipenem in Pseudomonas aeruginosa: clinical experience and biochemical mechanisms. Rev Infect Dis. 1988 Jul-Aug;10(4):892–898. doi: 10.1093/clinids/10.4.892. [DOI] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J Biol Chem. 1990 Sep 15;265(26):15680–15684. [PubMed] [Google Scholar]