Abstract
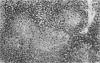
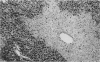
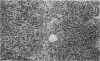
The present study extends previous reports of hepatic damage 24 h after halothane anaesthesia in the phenobarbitone pretreated hypoxic rat model by fully characterizing the lesion during the time course of its onset and recovery. Phenobarbitone treated animals exposed to halothane (1% for 2 h in 14% inspired oxygen) were killed 1, 2, 4, 6, 12 and 24 h and 2, 3, 5, 10, 15 and 30 days after commencement of the anaesthetic period. Blood was collected 1 day before the administration of halothane and at the time of killing for determination of serum alanine aminotransferase (ALT), a biochemical index of hepatic damage. Liver tissue was obtained immediately at post-mortem for histological examination. Serum ALT was increased at the end of the anaesthetic period, i.e. 2 h, with peak levels occurring at 12-24 h and remaining elevated for 3 days after exposure. Minor changes in liver histology were evident at 2 h in 50% of the animals and by 6 h all animals had mild hepatic injury. The extent of the necrosis was maximal at 24 h and this was sustained until 3 days. By 5 days after exposure minimal evidence of liver damage was observed and animals killed at 30 days had morphologically normal livers. Elevation of serum ALT or changes in liver histology were not observed in other treatment groups. The early onset of damage at 2-6 h is in keeping with direct hepatotoxicity associated with the biotransformation of halothane.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin S. B., Goodman Z. D., Ishak K. G., Zimmerman H. J., Irey N. S. The morphologic spectrum of halothane-induced hepatic injury: analysis of 77 cases. Hepatology. 1985 Nov-Dec;5(6):1163–1171. doi: 10.1002/hep.1840050617. [DOI] [PubMed] [Google Scholar]
- Fee J. P., Black G. W., Dundee J. W., McIlroy P. D., Johnston H. M., Johnston S. B., Black I. H., McNeill H. G., Neill D. W., Doggart J. R. A prospective study of liver enzyme and other changes following repeat administration of halothane and enflurane. Br J Anaesth. 1979 Dec;51(12):1133–1141. doi: 10.1093/bja/51.12.1133. [DOI] [PubMed] [Google Scholar]
- Gourlay G. K., Adams J. F., Cousins M. J., Sharp J. H. Time-course of formation of volatile reductive metabolites of halothane in humans and an animal model. Br J Anaesth. 1980 Mar;52(3):331–336. [PubMed] [Google Scholar]
- Hall P., Smith R. D., Gormley B. M. 'Routine' stains on osmicated resin embedded hepatic tissue. Pathology. 1982 Jan;14(1):73–74. doi: 10.3109/00313028209069044. [DOI] [PubMed] [Google Scholar]
- Hoft R. H., Bunker J. P., Goodman H. I., Gregory P. B. Halothane hepatitis in three pairs of closely related women. N Engl J Med. 1981 Apr 23;304(17):1023–1024. doi: 10.1056/NEJM198104233041707. [DOI] [PubMed] [Google Scholar]
- Jee R. C., Sipes I. G., Gandolfi A. J., Brown B. R., Jr Factors influencing halothane hepatotoxicity in the rat hypoxic model. Toxicol Appl Pharmacol. 1980 Feb;52(2):267–277. doi: 10.1016/0041-008x(80)90114-3. [DOI] [PubMed] [Google Scholar]
- Knights K. M., Gourlay G. K., Cousins M. J. Changes in rat hepatic microsomal mixed function oxidase activity following exposure to halothane under various oxygen concentrations. Biochem Pharmacol. 1987 Mar 15;36(6):897–906. doi: 10.1016/0006-2952(87)90182-1. [DOI] [PubMed] [Google Scholar]
- McLain G. E., Sipes I. G., Brown B. R., Jr An animal model of halothane hepatotoxicity: roles of enzyme induction and hypoxia. Anesthesiology. 1979 Oct;51(4):321–326. doi: 10.1097/00000542-197910000-00008. [DOI] [PubMed] [Google Scholar]
- Otsuka S., Yamamoto M., Kasuya S., Ohtomo H., Yamamoto Y., Yoshida T. O., Akaza T. HLA antigens in patients with unexplained hepatitis following halothane anesthesia. Acta Anaesthesiol Scand. 1985 Jul;29(5):497–501. doi: 10.1111/j.1399-6576.1985.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Peters R. L., Edmondson H. A., Reynolds T. B., Meister J. C., Curpey T. J. Hepatic necrosis associated with halothane anesthesia. Am J Med. 1969 Nov;47(5):748–764. doi: 10.1016/0002-9343(69)90168-5. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Weir D. M. Antibodies against the mitochondrial fraction of liver after toxic liver damage in rats. Clin Exp Immunol. 1966 Jan;1(1):33–43. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Paronetto F., Schaffner F., Popper H. Antimitochondrial antibodies in jaundice following drug administration. JAMA. 1969 Apr 7;208(1):148–150. [PubMed] [Google Scholar]
- Ross W. T., Jr, Daggy B. P., Cardell R. R., Jr Hepatic necrosis caused by halothane and hypoxia in phenobarbital-treated rats. Anesthesiology. 1979 Oct;51(4):327–333. doi: 10.1097/00000542-197910000-00009. [DOI] [PubMed] [Google Scholar]
- Vergani D., Mieli-Vergani G., Alberti A., Neuberger J., Eddleston A. L., Davis M., Williams R. Antibodies to the surface of halothane-altered rabbit hepatocytes in patients with severe halothane-associated hepatitis. N Engl J Med. 1980 Jul 10;303(2):66–71. doi: 10.1056/NEJM198007103030202. [DOI] [PubMed] [Google Scholar]
- Vergani D., Tsantoulas D., Eddleston A. L., Davis M., Williams R. Sensitisation to halothane-altered liver components in severe hepatic necrosis after halothane anaesthesia. Lancet. 1978 Oct 14;2(8094):801–803. doi: 10.1016/s0140-6736(78)92585-0. [DOI] [PubMed] [Google Scholar]