Abstract
Recombinant adenoviruses (Ads) are highly efficient at transferring foreign genes to the liver in vivo; however, the duration of gene expression is limited by the host antiviral immune response, which prevents expression upon readministration of the virus. To test whether overexpression of the immunomodulatory products of the early Ad genome region 3 (E3) could prevent the antiviral immune response and prolong expression of foreign genes delivered by Ad vectors, we injected a recombinant Ad (Ad-E3-hBUGT), containing both E3 and the human bilirubin-uridine-diphosphoglucuronate-glucuronosyltransferase (BUGT) genes, into BUGT-deficient hyperbilirubinemic Gunn rats. Control Gunn rats received Ad-hBUGT, which expresses human BUGT alone. An initial injection of either virus resulted in hepatic expression of human BUGT as evidenced by excretion of bilirubin glucuronides in bile and a reduction of mean serum bilirubin levels from 7.0 mg/dl to 1.9–2.7 mg/dl within 7 days. In Ad-E3-hBUGT-injected rats, serum bilirubin levels increased to 4.5 mg/dl by 84 days after infection, but a second administration of the virus on that day resulted in a hypobilirubinemic response similar to that seen with the first injection. In contrast, rats receiving Ad-hBUGT had serum bilirubin levels of 7 mg/dl on day 84 after infection, but showed no reduction of serum bilirubin by reinjection of the virus on that day. In the rats injected with Ad-E3-hBUGT, but not in the ones injected with Ad-hBUGT, there was a marked inhibition of the antiviral antibody and Ad-specific cytotoxic T lymphocyte responses. This is the first demonstration that insertion of E3 genes in recombinant Ads facilitates readministration of a functional vector for long-term correction of an inherited metabolic disorder.
Adenoviruses (Ads) are highly efficient at transferring foreign genes into nondividing cells (1). Ad serotype 5 (Ad 5) preferentially localizes in the liver after intravenous injection in rodents (2–4), making it an excellent vehicle for delivery of foreign genes to the liver. However, despite the high efficiency of gene transfer, foreign gene expression is transient because integration into the host cell genome is rare (5, 6). In addition, the viral proteins, as well as the potentially therapeutic gene product, induce cellular and humoral immune responses, thus precluding further gene expression after readministration of the virus (7–9).
The early transcription region (E1A) of the adenoviral genome controls viral gene expression and replication (5). First generation recombinant Ads were produced by inserting the target gene into the E1A/E1B region, thus disrupting the region and rendering the virus replication defective (3, 4). However, prolonged gene expression was difficult to achieve in vivo from these recombinant viruses, or even “second generation” vectors containing an additional mutation in the E2A region (10, 11). These results suggest that the initial viral antigenic stimulus was sufficient to induce a potent host immune response. Although systemic immunosuppression or tolerization of the host to adenoviral antigens permits long-term gene expression following repeated viral injections, these methods are either invasive or would require systemic immunosuppression, creating additional problems in the clinical setting (12–14). Therefore, an alternate approach is to engineer the virus itself to be less immunogenic, as we describe in this manuscript.
The approach to decrease the immune response specifically to the Ad vector used the Ad early region 3 (E3), which encodes several proteins that have the capacity to modulate the immune response of the host to Ad-infected cells (15, 16). This region of the Ad genome is not essential for viral replication and has been deleted from most of the currently used vectors to increase the potential size of packageable foreign DNA inserts (16). Moreover, because E3 region expression is dependent on the presence of the E1A region, it is not fully expressed from its natural promoter, even in vectors still containing E3 (5, 17). Of the seven known proteins that are encoded by the Ad-E3 region, a 19-kDa glycoprotein (gp19K) is known to inhibit transport of the major histocompatibility complex class I molecules to the cell surface, and thus to impair both peptide recognition and clearance of Ad-infected cells by cytotoxic T lymphocytes (CTLs) (18–20). In addition, there are three other gene products, a 14.7-kDa protein (14.7K) and the complex of 10.4- and 14.5-kDa proteins (10.4K and 14.5K), which control tumor necrosis factor α (TNFα) cytolysis of infected cells (reviewed in refs. 15 and 21).
The model of gene therapy that we have studied extensively is the mutant Gunn rat (12–14). Gunn rats lack hepatic bilirubin-uridine-diphosphoglucuronate-glucuronosyltransferase (BUGT) activity (22, 23). As a consequence, they do not excrete conjugated bilirubin in the bile. Gunn rats are an animal model of human Crigler–Najjar syndrome type I (24). Because glucuronidation is essential for hepatic disposition of bilirubin, Gunn rats and patients with Crigler–Najjar syndrome type I have lifelong unconjugated hyperbilirubinemia, resulting in brain damage (24, 25). We have previously shown that introduction of the gene for human BUGT (hBUGT) into Gunn rats, using a recombinant Ad vector, temporarily corrected the metabolic defect (12–14). However, virus reinjection to produce long-term therapeutic effects requires systemic immunosuppression, or the induction of tolerance by intrathymic or neonatal injection of viral antigens (12–14).
The results of our study demonstrate that co-insertion of the Ad E3 genes with the foreign gene (hBUGT) of interest facilitates long-term gene expression and correction of the metabolic defect by repeated injections of the virus. In addition to down-regulation of CTL, we have found, for the first time, that the E3 genes can greatly attenuate the antiviral humoral immune response.
MATERIALS AND METHODS
Generation of Ad-hBUGT and Ad-E3-hBUGT.
The recombinant Ad-hBUGT was generated from an Ad-5 based vector as described (12). For preparation of Ad-E3-hBUGT, the whole Ad-E3 region was cut out of the rat insulin II promoter (RIP)-E3 containing plasmid previously described (26), using BspeI and NdeI, and cloned into the EcoRV site of the pcDNA3 plasmid (Invitrogen). The expression cassette containing the cytomegalovirus (CMV) promoter and the E3 region was cut out with MunI and DraIII and cloned into the EcoRI–EcoRV site of the pΔE1sp1A plasmid (Microbix Biosystems, Toronto), leading to the ΔE1-CMV-E3 plasmid. The hBUGT gene was cloned into the XbaI–NotI site of pRc/CMV (Invitrogen). The expression cassette containing the hBUGT gene behind the CMV promoter was cut out using SmaI and NruI and cloned into the SalI site of ΔE1-CMV-E3, leading to the plasmid ΔE1-CMV-E3-CMV-hBUGT with the E3 and hBUGT present in reverse orientations. This plasmid with the double expression cassette (≈8.7 kb) and sequences from the left end of the Ad was cotransfected into 293 cells together with the pBHG10 plasmid (Microbix Biosystems), containing the rest of the Ad genome sequence needed to generate the virus Ad-E3-hBUGT by recombination. Ad-E3-hBUGT was plaque purified and both insertions were confirmed by PCR and Southern blotting (12, 26). Both recombinant Ads, as well as Ad-LacZ virus (obtained through the courtesy of M. Imperiale, University of Michigan, Ann Arbor), were grown on 293 cells in suspension culture as described (12).
Ad-hBUGT, Ad-E3-hBUGT, and Ad-LacZ Injections into Gunn Rats.
Four groups of Gunn rats (A–D), 10 animals in each group, were studied after intravenous injection of virus (5 × 1010 virions). Groups A and B were injected with Ad-E3-hBUGT on day 1 followed by a second Ad-E3-hBUGT injection (Group A) or Ad-LacZ (Group B) on day 84. Two groups were used as controls: Group C received two injections of Ad-hBUGT of the same dose given on the same days as Group A; Group D received Ad-hBUGT injection on day 1 followed by Ad-LacZ injection on day 84 (Table 1).
Table 1.
Experimental and control groups
| Group | First injection (day 1) | Second injection (day 84) |
|---|---|---|
| A | Ad-E3-hBUGT | Ad-E3-hBUGT |
| B | Ad-E3-hBUGT | Ad-LacZ |
| C | Ad-hBUGT | Ad-hBUGT |
| D | Ad-hBUGT | Ad-LacZ |
Expression of Human-BUGT and E3 Proteins.
The presence of the human BUGT gene in the rat liver was demonstrated by PCR assays as described (12, 13, 27). The expression of hBUGT and gp19K (as an example of an AdE3 protein) was measured in liver biopsies taken at various time intervals. Tissue homogenates (200 mg/ml) were prepared in 0.25 M sucrose/10 mM Tris·HCl (pH 7.4) using a glass homogenizer fitted with a motor-driven teflon pestle. For immunoblot analysis, proteins (100 μg per lane) were resolved by electrophoresis on SDS/7.5% polyacrylamide gels and electroblotted to nitrocellulose membranes. hBUGT protein was detected on the membranes with a monoclonal antibody, WP1, directed at the common carboxyl-terminal domains of UGT isoforms expressed by the hBUGT gene, followed by goat anti-mouse IgG Fab fragment second antibody (Sigma) coupled with horseradish peroxidase (27, 28). For detection of E3 expression, monoclonal antibody to the gp19K was used as the primary antibody, followed by a peroxidase-conjugated rabbit anti-mouse antibody. Ad-E3-hBUGT virus-infected 293 cells were used as a positive control.
Evaluation of the Anti-Adenoviral Immune Response. Adenoviral antibodies.
Anti-Ad neutralizing antibodies in the sera of rats were measured on days 28, 98, and 132 as described (12, 13). Anti-Ad antibodies were also measured by ELISA in 96-well plates coated with 1 × 108 particles per well of Ad-E3-BUGT in PBS at 4°C overnight. The wells were washed five times with PBS-Tween, blocked with 3% BSA in PBS, washed again, and incubated for 2 hr with serial dilutions of the sera (in 1% BSA) at 37°C. IgG levels were measured after 0.1 M 2-mercapthoethanol pretreatment of the sera for 1 hr at 37°C, to dissociate and denature IgM (29). The wells were washed and incubated with 100 μl of a 1:1000 dilution of alkaline phosphatase-conjugated goat anti-rat IgG, IgA, or IgM (Bethyl Laboratories, Montgomery, TX), for 2 hr at 37°C, washed, and developed with substrate (104 Phosphate Substrate, Sigma). Plates were read at 405 nm in an ELISA reader. Two negative control sera from naive Gunn rats were included in each plate. Endpoint titers were expressed as the reciprocal of the last sample dilution, which gave 2-fold greater absorbance than the negative controls.
CTL assay.
CTL directed against Ad (E3 deleted)-infected hepatocytes were prepared from the spleen, restimulated in vitro, and assayed by measuring alanine aminotransferase (ALT) levels released from Ad-infected primary hepatocytes as target cells. CTL activity was expressed in units of ALT [measured with a kit (Sigma)] averaged from 6 wells after subtraction of background levels as described (12).
RESULTS
Rats Injected with Ad-E3-hBUGT Do Not Develop Anti-Adenoviral Antibodies. Neutralizing antibodies.
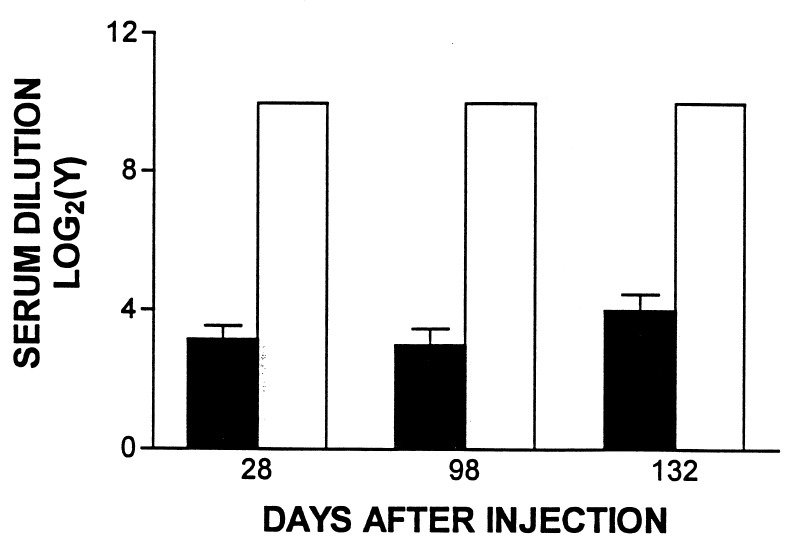
After injection of Ad-hBUGT in control rats (Group C, see Table 1), all animals developed high titer (>1:1024) antibodies by 28 days p.i. These titers remained elevated when measured on day 98 (14 days after the second virus injection) or on day 132. In contrast, in the Ad-E3-hBUGT-injected rats (Group A), neutralizing antibodies were undetectable in 4 of the 9 recipients, and the remainder exhibited very low antibody titers (≈1:8) (Fig. 1), which did not affect their hypobilirubinemic response after the second injection of Ad-E3-hBUGT (see below). Titers in this group of animals did not increase significantly after the second virus injection on day 84.
Figure 1.
Anti-Ad neutralizing antibody levels in Ad-E3-hBUGT- and Ad-hBUGT-treated rats. Antibody levels were measured in sera from nine rats in Group A (Ad-E3-hBUGT, solid bars) and seven control rats from Group C (Ad-hBUGT, open bars), 28 days after the first virus injection. In Group A, four animals had no detectable titers in undiluted sera but were included in the calculations of the average and the standard deviation. In Group C, all animals had titers greater than/or equal to 1:1024, which was the highest dilution of serum tested. Corresponding values are shown for day 98 (eight animals in Group A, four with undetectable titers; five animals in Group C) and for day 132 (eight animals in Group A, four with undetectable titers; seven animals in Group C).
Anti-Ad antibodies by ELISA.
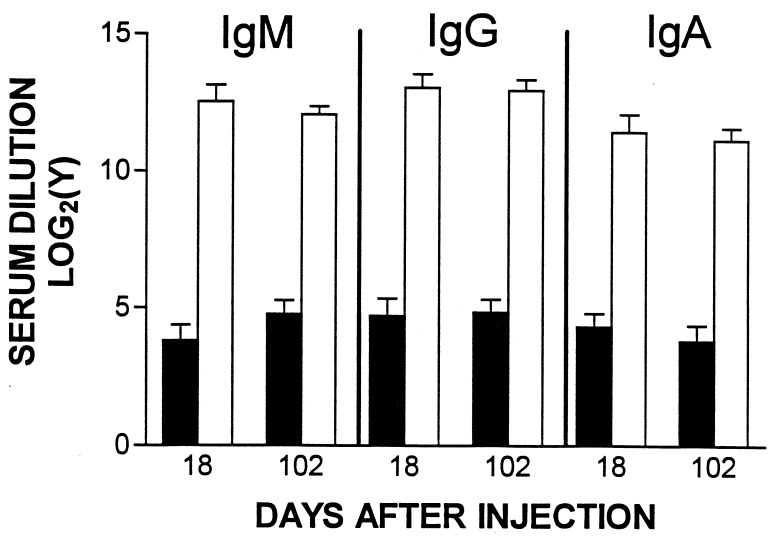
To determine whether nonneutralizing antibodies (30) were formed after Ad-E3-hBUGT infection, four to six rats from Groups A and C were tested for serum antiadenoviral IgG, IgM, and IgA antibodies on days 18 and 102. At both time points, the titer of anti-Ad antibodies of each class was low (1:16–1:32) in Ad-E3-hBUGT-treated rats (Group A). In contrast, high-titer (1:4,096–1:16,384) antibodies of all three isotypes were found after each injection in Ad-hBUGT-treated rats (Group C) (Fig. 2). We also wanted to measure whether animals that did not make antibodies to Ad-E3-hBUGT could subsequently make antibodies to Ads of the same serotype that do not contain E3. Thus, sera of rats from both Groups B and D were tested on day 112 after Ad-LacZ injection. These rats showed high titers of antibodies (210-213; data not shown), indicating that prior injection of Ad-E3-hBUGT did not prevent a subsequent antibody response after Ad-LacZ injection.
Figure 2.
Anti-Ad ELISA levels in Ad-E3-hBUGT- and Ad-hBUGT-treated rats. Antibody levels (IgM, IgG, and IgA) were measured in sera from 4–5 rats in Group A (Ad-E3-hBUGT, solid bars) and 5–6 control rats from Group C (Ad-hBUGT, open bars) on day 18, after the first virus injection and on day 102, after the second virus injection. Averages and standard deviations for all animals are shown for each group. (There was a single animal in Group A that had no detectable IgM, IgG, and IgA antibodies on day 18, and a different Group A animal that lacked all three antibodies on day 102).
Rats Injected with Ad-E3-hBUGT Have an Attenuated Ad-Specific CTL Response and Little Evidence of Hepatic Inflammation.
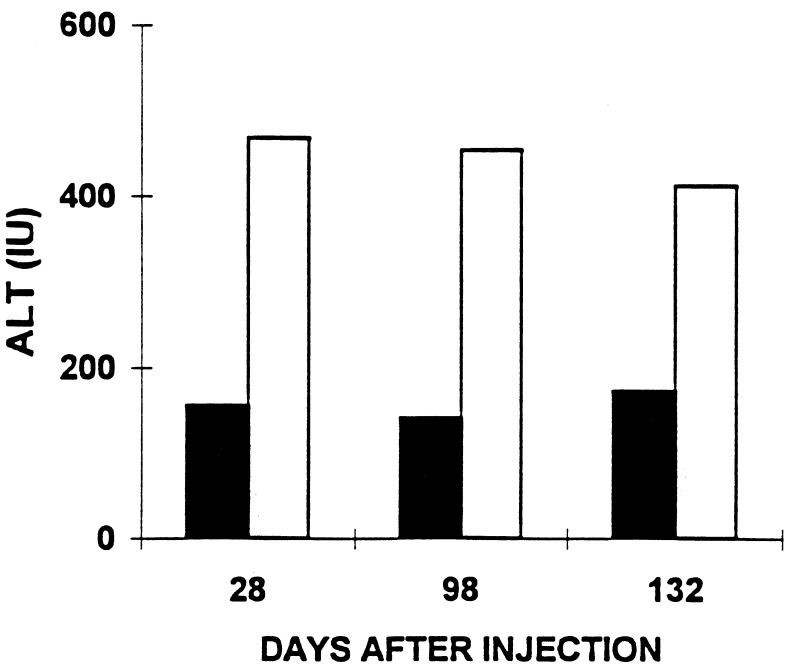
CTL response against Ad-infected hepatocytes was evaluated three times during this study. Damage to target cells by splenic lymphocytes restimulated in vitro was evaluated by ALT release in the media (see Materials and Methods). Lymphocytes from Ad-hBUGT-injected rats resulted in the release of 469 units of ALT into the media (Fig. 3). In contrast, lymphocytes from animals infected with Ad-E3-hBUGT induced the release of less than 173 units of ALT. This represents nearly a 3-fold reduction in CTL activity, which probably is a function of the Ad E3 gp19K (anti-major histocompatibility) gene function. Liver biopsies from animals infected with the Ad vector containing E3 genes also demonstrated a decrease in the inflammatory response when compared with animals infected with E3 negative virus. Liver biopsies obtained from two rats in Group A, taken 7 days after the second injection, showed minimal or no periportal or lobular lymphocytic infiltration. However, there was periportal lymphocytic infiltration in the lobules with “piecemeal necrosis” observed 7 days after the second injection, in liver specimens of rats that received Ad-hBUGT (Group C) (data not shown).
Figure 3.
Effect of Ad-E3-hBUGT tolerization on CTLs. CTLs were assayed in Ad-E3-hBUGT-treated rats (Group A, solid bars) and control Ad-hBUGT-treated rats (Group C, open bars). T cells were harvested on days 28, 98, and 132 from two rats in each group and were used as effector cells against Ad-infected primary hepatocytes. CTL killing was expressed as ALT levels released from Ad-infected hepatocytes as described.
Rats Injected with Ad-E3-hBUGT, but Not Ad-hBUGT, Express Functional hBUGT and Viral Gene Products After Reinjection of the Virus.
To determine that the inhibition of antibody formation to Ads and the modulation of Ad-induced CTL were caused by functional Ad E3 genes, a variety of assays were used to determine these viral products.
DNA analysis using PCR.
The presence of hBUGT DNA in the liver of Gunn rats from Groups A and C (Table 1) was tested by PCR on day 91. A DNA fragment of 321 bp was seen only in rats from Group A (Ad-E3-hBUGT), whereas the control Group C (Ad-hBUGT) was negative. Normal human liver and liver from an untreated Gunn rat were used as positive and negative controls, respectively (Fig. 4).
Figure 4.
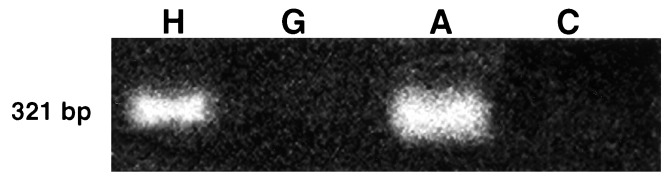
The presence of the hBUGT gene in rat liver after the second administration of Ad-E3-hBUGT virus. Liver specimens, from rats in Groups A and C were obtained 7 days after the second recombinant virus injection (day 91 of the experiment), and tested by PCR for the presence of the 321-bp band of hBUGT DNA (see Materials and Methods). Positive control, normal human liver (H); negative control, untreated Gunn rat (G).
Expression of human-BUGT and E3 proteins.
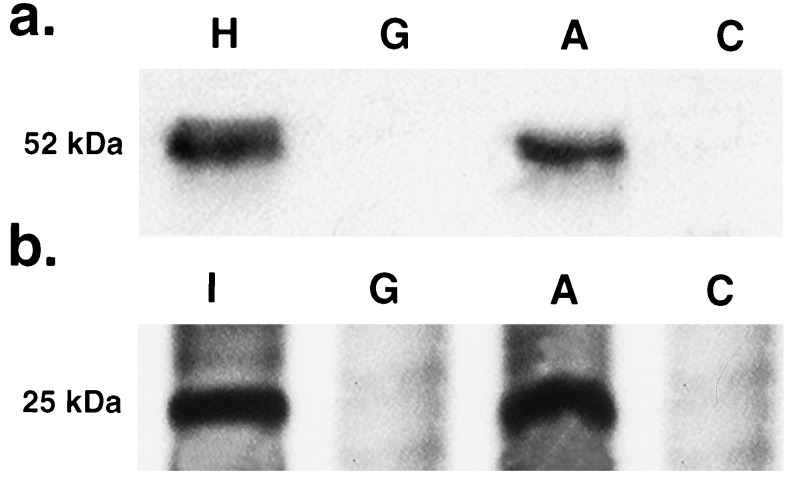
Liver specimens were collected from two rats in each group (A and C) on day 91, 7 days after the second virus injection. Immunoreactive 52-kDa bands, corresponding to hBUGT, were observed in the treated rats in Group A following the second injection, but not in rats from the control Group C (Fig. 5). Normal human liver and untreated Gunn rat livers were used as positive and negative controls, respectively. Similarly, a positive 25-kDa band corresponding to a glycosylated form of the E3-encoded gp19K protein was observed only in rats from Group A and not in the control (Group C) rats.
Figure 5.
(a) Expression of hBUGT protein on day 91 in liver homogenates from a representative rat in Group A compared with a rat from Group C. The positive control was normal human liver (H); the negative control was untreated Gunn rat (G). A 52-kDa band is seen only in the liver of the rat from Group A. (b) Expression of the gp19K-E3 protein in liver from a rat in Group A compared with a rat from Group C. The positive control was 293 cells transfected with the gp19K (I); the negative control was a liver specimen from an untreated Gunn rat (G). A 25-kDa band representing the glycosylated size of gp19K is seen in the specimen from Group A but not Group C.
BUGT activity.
The in vivo correlation of expression of hBUGT from an Ad-transfected gene was studied to measure the efficacy of the Ad E3-induced immunosuppression of target recognition on the expression of a transfected gene. BUGT activity using bilirubin substrate and liver homogenates was undetectable in untreated Gunn rats. In homogenates of normal human specimens obtained from cadaver donor organs, the BUGT activity was 81 ± 22 nmol/mg liver weight per min (mean ± SEM, n = 3). In liver homogenates from two rats in Group A, BUGT activity was 84 and 79 nmol/mg liver weight per min 7 days after the second Ad-hBUGT injection. In control Group C, BUGT activity after the second injection was undetectable when measured as described (31, 32).
Serum bilirubin levels.
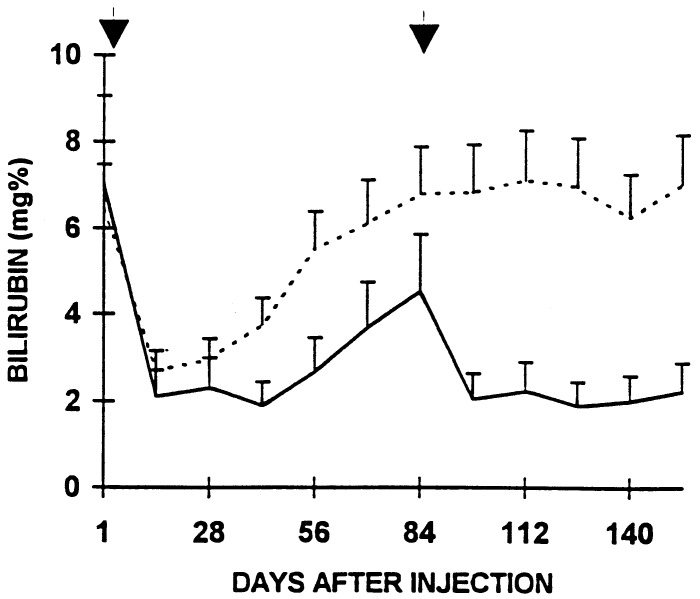
Bilirubin levels were measured every 7–14 days as described (31, 32). Both viruses caused an initial reduction of mean serum bilirubin levels from 7.0 mg/dl to 1.9–2.7 mg/dl in 7 days. In Ad-E3-hBUGT-injected rats, the reduction in serum bilirubin levels was maintained for 35 days, after which the levels progressively increased to 4.5 ml/dl serum by day 84. A repeat injection of the Ad-E3-hBUGT virus on day 84 resulted in a rapid decrease in serum bilirubin similar to that seen with the first injection (Fig. 6). In rats receiving Ad-hBUGT, serum bilirubin levels began to increase within 28–35 days, but more significantly were not reduced by reinjection of the virus on day 84 (Fig. 6).
Figure 6.
Effect of E3 expression on serum bilirubin levels in rats from Group A (solid line) that received two Ad-E3-hBUGT injections (days 1 and 84). Control rats (broken line) from Group C received Ad-hBUGT injections on the same days.
Bile pigment analysis.
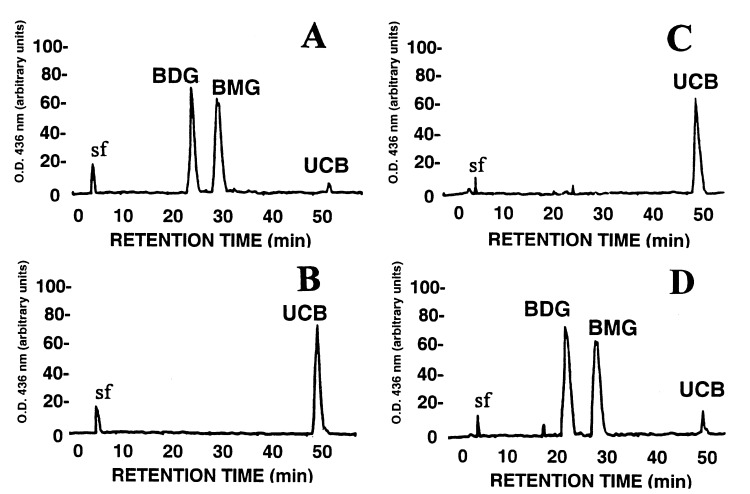
HPLC analysis, as previously described (32), of bile collected from two rats in Group A, 21 days after the second injection (day 105), showed excretion of bilirubin monoglucuronide and diglucuronide. The two glucuronides accounted for more than 95% of the bile pigments, less than 5% being unconjugated bilirubin. This profile is similar to that seen in normal Wistar rats. In contrast, in control Group C, bile pigment analysis following the second injection showed no significant conjugated bilirubin excretion in the bile (Fig. 7).
Figure 7.
HPLC analysis of bile pigments. Bile was collected by cannulation of the bile ducts of rats from Groups A and C, 21 days after the second virus injection. (A) A normal Wistar rat bile analysis profile where greater than 95% of the bilirubin in the bile is conjugated. (B) Bile analysis of an untreated Gunn rat in which all of the bilirubin in bile is unconjugated. (C) Bile analysis from a control rat from Group C after the second virus injection. The vast majority of the bilirubin is unconjugated. (D) Analysis of bile in a rat from Group A after the second injection showing that most of the bilirubin is conjugated. BMG, bilirubin monoglucuronide; BDG, bilirubin diglucuronide; UCB, unconjugated bilirubin.
Expression of β-galactosidase activity.
We used the Ad-LacZ virus to challenge Ad-E3-hBUGT-injected animals to confirm that the liver could be infected by a subsequent administration of Ad. Administration of this virus also determined whether the animals could mount a normal immune response after temporary induction of immune “ignorance” after injection of E3-containing Ad. Cryostat sections (10 μm) were prepared on day 91 from liver specimens of two rats in Groups B and D, 7 days after Ad-LacZ injection. Nearly all hepatocytes stained positive for β-galactosidase activity after the injection in Group B rats. In contrast, only 5% of hepatocytes were positive in livers from rats in Group D (data not shown). These data indicate that animals in Group B have an attenuated primary antiadenoviral immune response that permits successful expression of Ad genes, but also have a normal immune response after reinjection of an E3-negative virus.
DISCUSSION
The practical application of gene therapy requires safe methods that allow long-term expression of the therapeutic gene product. Although retroviral vectors partially fulfill this requirement, their inability to integrate into nondividing cells makes them inconvenient for gene transfer to the liver in vivo, as hepatocytes undergo mitosis only infrequently (33). Our previous results showed that recombinant Ad vectors are capable of transducing the great majority of hepatocytes in vivo permitting correction of the metabolic defect in Gunn rats (12). However, the limitations of Ad vectors are the relatively short duration of gene expression and the prompt development of a potent host anti-Ad immune response that prevents transgene expression upon subsequent injections of virus (7–11).
The anti-Ad immune response consists of both humoral and cell-mediated components (8). The production of neutralizing antibodies to the Ad has been correlated with the failure of gene expression when the virus is readministered after successful primary infection (8–11). In addition, loss of gene expression, as well as hepatic inflammation, appears to be mediated in part by a cellular immune response against Ad-infected cells (9). The role of the T cell immune system in clearing Ad from infected rats was shown by studies in which continuous administration of cyclosporine, or short-term FK506 treatment, both of which inhibit interleukin 2 production, suppressed the proliferation of activated T cells and resulted in the prolongation of gene expression (14, 34). Use of knockout mice deficient in either the humoral or cellular arm of the immune system has shown that both processes contribute to the antiviral response, and that prolonged gene expression following systemic administration of virus is only satisfactorily achieved with profound immunodeficiency, such as is present in severe combined immunodeficient mice (7, 9). Inhibition of both arms of the immune response by systemic immunosuppression has permitted long-term gene expression using Ad vectors (14, 34). In addition, we have previously shown that the induction of tolerance by injection of recombinant virus in newborns (12), or by intrathymic or oral administration of major viral structural proteins (13, 35), resulted in long-term gene expression by repeated injection of Ad vectors. However, because most of these methods are invasive or impractical for clinical use and may result in suppression of the immune response related to wild-type viruses, an alternate approach that modifies the vector itself to make it less immunogenic would be preferred for the future use of these vectors in humans. In pursuit of these goals, we have overexpressed the immunomodulatory functions of the Ad-E3 region and successfully controlled the humoral and cellular immune responses toward injected Ad gene therapy vectors.
We have previously used the immunomodulatory functions of the Ad E3 genes to facilitate allogeneic cell transplantation. Ad E3 proteins under the control of the RIP, were expressed in pancreatic β cells of transgenic mice. The RIP-E3 containing islet allografts survived for more than 94 days under the kidney capsule of recipient mice in contrast with controls, which were rejected in fewer than 30 days (36). We also have shown that the same RIP-E3 transgenes can prevent the onset of auto-immune diabetes in the lymphocytic choriomeningitis virus-induced diabetes model, in which transgenic mice contain a lymphocytic choriomeningitis virus protein expressed behind the RIP (M. G. Von Herrath, S. Efrat, M. B. A. Oldstone, and M.S.H., personal communication). We therefore hypothesized that E3-mediated functions could similarly be harnessed to suppress the anti-Ad immune response. The data presented in this paper conclusively demonstrate that overexpression of the E3 proteins, as exemplified by measuring gp19K expression, modulates the anti-Ad immune response in rats, and permits long-term gene expression that effectively corrects the metabolic defect in Gunn rats.
Two previous studies have reported the use of E3-encoded proteins to suppress an anti-Ad immune response. Recombinant Ad expressing the gp19K protein alone, under the control of a Rous sarcoma virus promoter, has been reported to down-regulate the antiviral CTL response (20). In the latter study, however, neither prolongation of gene expression nor the effect on the humoral immune response were studied (20). In a second recent report, it was demonstrated that injection of an Ad containing the intact E3 region together with factor IX behind a strong promoter led to prolonged expression of this clotting factor in genetically deficient mice (37). However, the latter study used adjuvant therapy with anti-CD4 serum, and there was no information regarding the production of antiviral antibodies in these animals (37). In our study, we have shown conclusively that injection of Ad overexpressing E3-encoded gene products results not only in inhibition of cytotoxic activity toward Ad-infected cells, but also in marked down-regulation of antibody formation to structural viral proteins. Since the induction of neutralizing antibodies to Ad will prevent infection of target cells upon readministration of the virus, this approach facilitates repeated viral administration without the need for systemic immunosuppression.
Genes encoded by the E3 region inhibit multiple pathways of the antiviral immune response. Down-regulation of surface major histocompatibility complex class I expression, mediated by gp19K, interferes with presentation of viral peptides and subsequent induction of CD8+ cytotoxic T lymphocytes, which mediate lysis of virally infected cells (15, 21). Lysis might further be inhibited by the effect on TNFα activity by other E3-encoded proteins (15). Thus, the absence of Ad-specific CTL in animals injected with Ad-E3-hBUGT was expected. However, the surprising feature of our study was the profound inhibition of the antibody response to E3-containing virus. This inhibition may be mediated by several mechanisms affecting B or T cells, individually or together. One possible explanation is that the liver, especially in rodents, has a very high affinity for Ad5, and once internalized, in the absence of CTL- or TNFα-induced cytolysis, there may be no antigen released for presentation by antigen-presenting cells or dendritic cells to initiate an antibody response. Such a formulation is consistent with some of the data derived from β2-microglobulin minus (β2mnull) mice, which lack class I major histocompatibility complex at the cell surface and do not develop CTL effector cells. However, their TNFα dependent mechanisms appear to be intact. When β2mnull mice were infected with vaccinia virus, they manifested similar disease and recovery patterns as wild-type controls, but failed to make a significant IgG antibody response to the invading virus (38). However, in contrast to our rats challenged with Ad-E3-hBUGT, the β2mnull mice did make IgM in response to the infectious agent (38). Our data, that nonneutralizing antibody including IgM is also not made in Ad-E3-hBUGT-injected animals is consistent with an earlier processing defect of antigen. Because TNFα is one of the cytokines that controls dendritic cell maturation and migration, early antigen presentation may be down-regulated in our model by the inhibition of this cytokine by E3 proteins. This explanation is also consistent with our finding that injection of Ad-LacZ into animals that previously did not mount an antibody response to Ad-E3-hBUGT, resulted in induction of a high titer antibody response. This demonstrates that the Ad-E3-hBUGT-injected animals have not developed tolerance to the virus, but rather that they manifest immune “ignorance.”
In summary, this study shows that the use of recombinant Ad vectors containing the E3 genes enables safe, long-term, and effective foreign gene expression without the need for systemic immunosuppression. We also show that the presence of the E3 genes and presumably one or more of the proteins they encode have the capacity to inhibit the antiviral humoral immune response. This effect is due to decreased immunogenicity of the virus and does not involve immune tolerance, which might subsequently prevent an immune response to wild-type virus. The exact mechanism and the protein(s) responsible for these effects are not yet understood. Further studies using Ad mutants lacking selected parts of the E3 region may help to solve this problem.
Acknowledgments
This work was supported in part by National Institutes of Health Grants ROI-DK 46057 (to J.R.C.), RO1-DK 39137 (to N.R.C.), and P30-DK 41296 (to the Liver Research Core Center). M.S.H. was supported by National Institutes of Health Grant RO1-CA69703 and Cancer Center Core Grant CA13330 of the Albert Einstein College of Medicine. Y.I. was supported by National Institutes of Health Hepatology Training Grant T32DK07218.
ABBREVIATIONS
- Ad
adenovirus
- BUGT
bilirubin-uridine-diphosphoglucuronate-glucuronosyltransferase
- ALT
alanine aminotransferase
- CTL
cytotoxic T lymphocytes
- E3
adenovirus early region three
- TNFα
tumor necrosis factor α
- RIP
rat insulin II promoter
References
- 1.Ali M, Lemoine N R, Ring C J A. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- 2.Jaffe H A, Danel C, Longenecker G. Nat Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 3.Prevec L, Schneider M, Rosenthal K L, Belbeck L W, Derbyshire J B, Graham F L. J Gen Virol. 1989;70:429–434. doi: 10.1099/0022-1317-70-2-429. [DOI] [PubMed] [Google Scholar]
- 4.Graham F L, Prevec L. In: Methods in Molecular Biology. Murray E J, editor. Clifton, NJ: Humana; 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz M S. In: Virology. Fields B N, Knipe D M, editors. New York: Raven; 1990. pp. 1679–1721. [Google Scholar]
- 6.Bett A J, Prevec L, Graham F L. J Virol. 1993;67:5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armentano D, Thompson A R, Darlington G, Woo S L. Proc Natl Acad Sci USA. 1990;87:6141–6145. doi: 10.1073/pnas.87.16.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Li Q, Ertl H C J, Wilson J M. J Virol. 1995;67:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Y, Schwarz E M, Gu D, Zhang W W, Sarvetnick N, Verma I M. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi M, Ilan Y, Guida J, Horwitz M, Roy Chowdhury N, Roy Chowdhury J. J Biol Chem. 1996;271:26536–26542. doi: 10.1074/jbc.271.43.26536. [DOI] [PubMed] [Google Scholar]
- 13.Ilan Y, Attavar P, Takahashi M, Davidson A, Horwitz M, Guida J, Roy Chowdhury N, Roy Chowdhury J. J Clin Invest. 1996;98:2640–2647. doi: 10.1172/JCI119085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilan Y, Jona V K, Prakash R, Sengupta K, Horwitz M, Roy Chowdhury N, Roy Chowdhury J. Hepatology. 1996;24:138. doi: 10.1002/hep.510260422. (abstr.). [DOI] [PubMed] [Google Scholar]
- 15.Wold W S, Tollefson A E, Hermiston T W. Curr Top Microbiol Immunol. 1995;199:237–274. doi: 10.1007/978-3-642-79496-4_13. [DOI] [PubMed] [Google Scholar]
- 16.Bett A J, Haddara W, Prevec L, Graham F L. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabner J J, Couture L A, Gregory R, Graham S M, Smith A E, Welsh M J. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 18.Feuerbach D, Burgert H G. EMBO J. 1993;12:3153–3161. doi: 10.1002/j.1460-2075.1993.tb05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beier D C, Cox J H, Vining D R, Cresswell P, Engelhard V H. J Immunol. 1994;22:3862–3872. [PubMed] [Google Scholar]
- 20.Lee M G, Abina M A, Haddada H, Perricaudet M. Gene Ther. 1995;2:256–262. [PubMed] [Google Scholar]
- 21.Horwitz M S, Tufariello J, Grunhaus A, Fejer G. Curr Top Microbiol Immunol. 1995;199:195–211. doi: 10.1007/978-3-642-79586-2_10. [DOI] [PubMed] [Google Scholar]
- 22.Roy Chowdhury J, Roy Chowdhury N, Falancy C N, Tephly R R, Arias I M. Biochem J. 1986;233:827–837. doi: 10.1042/bj2330827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy Chowdhury J, Novikoff P M, Roy Chowdhury N, Novikoff A B. Proc Natl Acad Sci USA. 1985;82:2990–2994. doi: 10.1073/pnas.82.9.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crigler J F, Najjar V A. Pediatrics. 1952;10:169–180. [PubMed] [Google Scholar]
- 25.Bosma P J, Roy Chowdhury N, Goldhoom B G, Hofker M H, Elferink T, Jansen P L M, Roy Chowdhury J. Hepatology. 1992;15:941–947. doi: 10.1002/hep.1840150531. [DOI] [PubMed] [Google Scholar]
- 26.Fejer G, Gyory I, Tufariello J, Horwitz M S. J Virol. 1994;68:5871–5881. doi: 10.1128/jvi.68.9.5871-5881.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters W H M, Alloebes P L M, Jansen L G, Capel P J A. Gastroenterology. 1987;93:162–169. doi: 10.1016/0016-5085(87)90329-5. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott D W, Gershon R K. Clin Exp Immunol. 1970;6:313–316. [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz M S. In: Virology. Fields B N, Knipe D M, Howley P M, editors. New York: Raven; 1996. pp. 2149–2171. [Google Scholar]
- 31.Trotman B W, Roy Chowdhury J, Wirt G D. Anal Biochem. 1982;121:175–180. doi: 10.1016/0003-2697(82)90572-3. [DOI] [PubMed] [Google Scholar]
- 32.Roy Chowdhury J, Roy Chowdhury N, Wu G, Shouval R, Arias I. Hepatology. 1981;1:622–627. doi: 10.1002/hep.1840010610. [DOI] [PubMed] [Google Scholar]
- 33.Wilson J M, Jefferson D M, Roy Chowdhury J, Novikoff P M, Johnston D M, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kass-Eisler A, Falck-Pederson E, Elfenbein D H, Alvira M, Buttrick P M, Leinwand L A. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 35.Ilan, Y., Roy Chowdhury, N., Prakash, R., Jona, V. K., Droguett, G., Horwitz, M., Davidson, A. & Roy Chowdhury, J. (1997) J. Clin. Invest., in press. [DOI] [PMC free article] [PubMed]
- 36.Efrat S, Fejer G, Brownlee M, Horwitz M. Proc Natl Acad Sci USA. 1995;92:6947–6951. doi: 10.1073/pnas.92.15.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polier W, Schneider-Rasp S, Liebert U, Merklein F, Thalheimer P, Haack A, Schwaab R, Schmitt C, Brackmann H H. Gene Ther. 1996;63:521–530. [PubMed] [Google Scholar]
- 38.Spriggs M K, Koller B H, Sato T, Morrisey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszweski C R. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]