Abstract
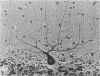
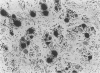
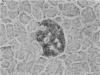
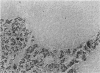
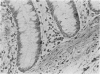
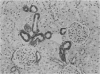
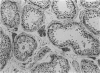
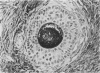
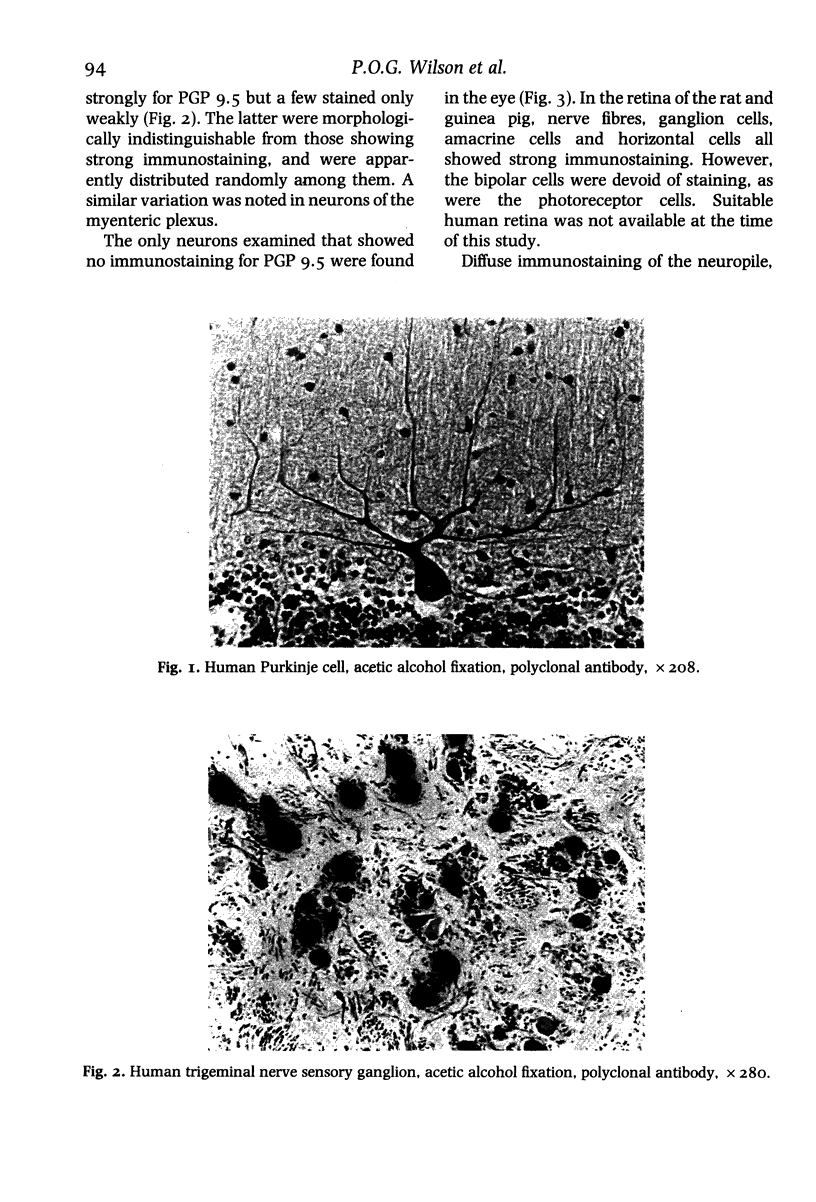
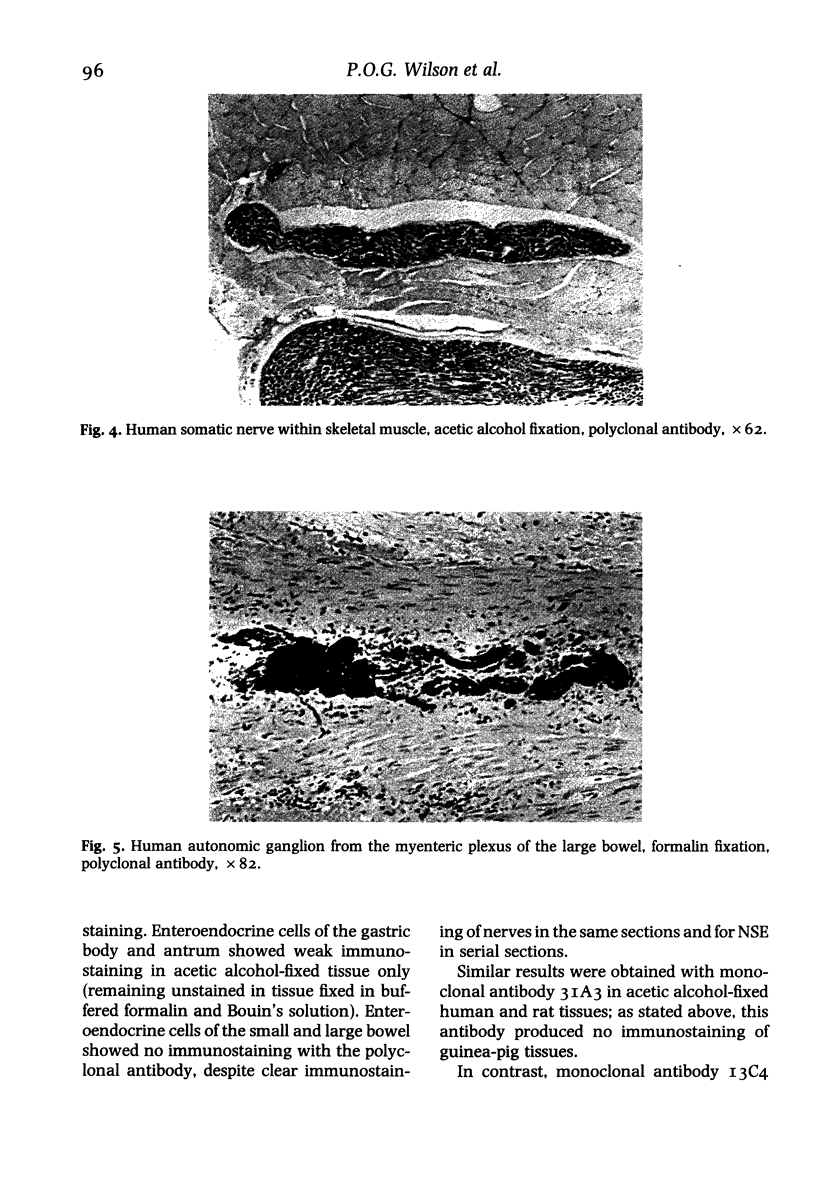
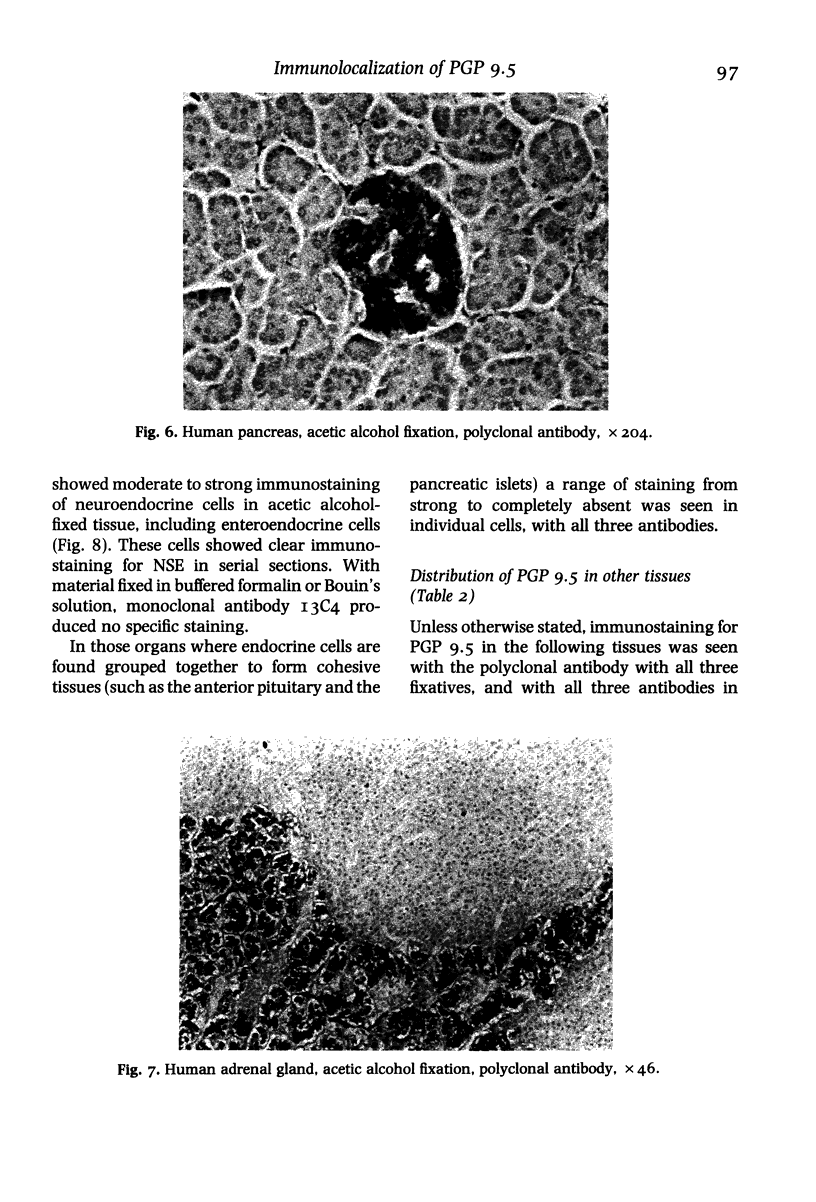
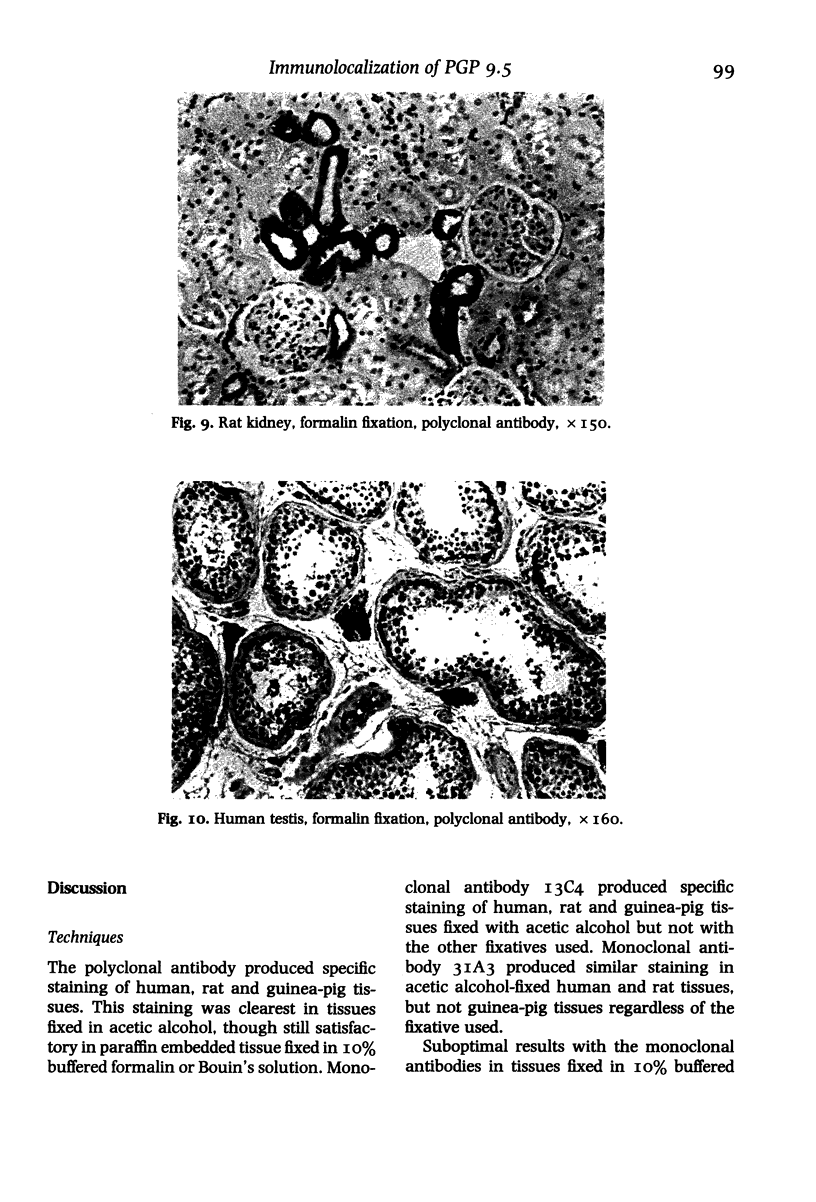
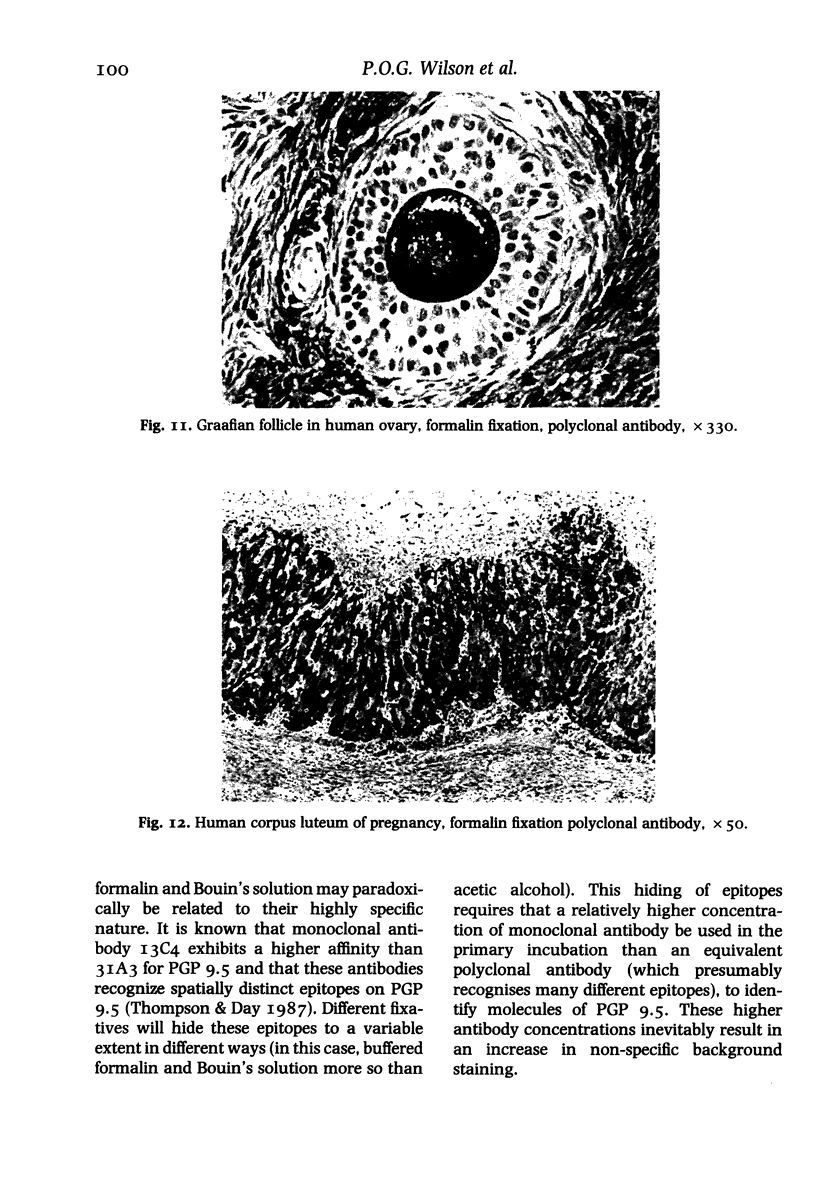
In order to assess the potential of protein gene product (PGP) 9.5 as a marker of the nervous and neuroendocrine systems, we examined its immunolocation in human, rat and guinea-pig tissues, using a rabbit polyclonal antiserum and two new mouse monoclonal antisera, I3C4 and 3IA3. Our results demonstrate immunoreactive PGP 9.5 in neurons and nerve fibres at all levels of the central and peripheral nervous system, in many neuroendocrine cells, in part of the renal tubule, in spermatogonia and Leydig cells of the testis, and in ova and in some cells of the pregnant and non-pregnant corpus luteum. In routinely processed tissues, standard immunohistochemical techniques using the polyclonal antibody demonstrated peripheral nerve fibres of all sizes with striking clarity.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop A. E., Polak J. M., Facer P., Ferri G. L., Marangos P. J., Pearse A. G. Neuron specific enolase: a common marker for the endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982 Oct;83(4):902–915. [PubMed] [Google Scholar]
- Bradbury J. M., Thompson R. J. Immunoassay of the neuronal and neuroendocrine marker PGP 9.5 in human tissues. J Neurochem. 1985 Feb;44(2):651–653. doi: 10.1111/j.1471-4159.1985.tb05461.x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Haimoto H., Takahashi Y., Koshikawa T., Nagura H., Kato K. Immunohistochemical localization of gamma-enolase in normal human tissues other than nervous and neuroendocrine tissues. Lab Invest. 1985 Mar;52(3):257–263. [PubMed] [Google Scholar]
- Haimoto H., Takashi M., Koshikawa T., Asai J., Kato K. Enolase isozymes in renal tubules and renal cell carcinoma. Am J Pathol. 1986 Sep;124(3):488–495. [PMC free article] [PubMed] [Google Scholar]
- Huang S. N., Minassian H., More J. D. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976 Oct;35(4):383–390. [PubMed] [Google Scholar]
- Ludwin S. K., Kosek J. C., Eng L. F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- Rode J., Dhillon A. P., Doran J. F., Jackson P., Thompson R. J. PGP 9.5, a new marker for human neuroendocrine tumours. Histopathology. 1985 Feb;9(2):147–158. doi: 10.1111/j.1365-2559.1985.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature. 1978 Dec 21;276(5690):834–836. doi: 10.1038/276834a0. [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Zis A. P., Brightman M., Goodwin F. K. Brain endolases as specific markers of neuronal and glial cells. Science. 1978 Jan 20;199(4326):313–315. doi: 10.1126/science.339349. [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Doran J. F., Jackson P., Dhillon A. P., Rode J. PGP 9.5--a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983 Nov 14;278(1-2):224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Walkenstein N., Lee V. M. Expression of neurofilament subunits in neurons of the central and peripheral nervous system: an immunohistochemical study with monoclonal antibodies. J Neurosci. 1986 Mar;6(3):650–660. doi: 10.1523/JNEUROSCI.06-03-00650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H. [Studies on the immunohistochemical localization of S-100 and glial fibrillary acidic proteins in the rat nervous system and in human brain tumors (author's transl)]. No To Shinkei. 1980 Oct;32(10):1055–1064. [PubMed] [Google Scholar]