Abstract
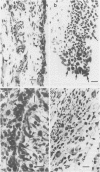
The activity and distribution of the collagenolytic lysosomal enzyme, cathepsin L, has been assessed in the synovial lining of the rabbit during the initiation and development of chronic (antigen-induced) arthritis. Biochemical assay of homogenates of synovial lining revealed a marked increase in the activity of lysosomal enzymes, including cathepsin L, between 1 and 5 days following initiation of arthritis. Elevated levels of these enzymes were still present at 4 weeks duration of arthritis. Using a monospecific antibody to cathepsin L. enzyme was immunolocated only in synovial lining cells in normal joints. In arthritic joints the enzyme was found in synovial lining cells, synovial fibroblasts and in infiltrating macrophages. Measurement, by scanning and integrating microdensitometry, of the peroxidase-reaction product in synovial lining cells revealed an increased content of cathepsin L in these cells in the diseased tissues.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consden R., Doble A., Glynn L. E., Nind A. P. Production of a chronic arthritis with ovalbumin. Its retention in the rabbit knee joint. Ann Rheum Dis. 1971 May;30(3):307–315. doi: 10.1136/ard.30.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T. Aetiological factors in the collagen diseases. Lysosomal enzymes and the degradation of cartilage matrix. Proc R Soc Med. 1962 Feb;55:109–111. [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E., Chayen J. Experimental allergic arthritis in the rabbit: alterations in the cellularity and the rate of cellular proliferation in the synovial linings of the challenged joints of rabbits immunized with antigen in Freund's incomplete adjuvant. Br J Exp Pathol. 1982 Feb;63(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Glynn L. E. Metabolic alterations in the synoviocytes in chronically inflamed knee joints in immune arthritis in the rabbit: comparison with rheumatoid arthritis. Br J Exp Pathol. 1981 Feb;62(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- Henderson B. The contribution made by cytochemistry to the study of the metabolism of the normal and rheumatoid synovial lining cell (synoviocyte). Histochem J. 1982 Jul;14(4):527–544. doi: 10.1007/BF01011886. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Taylor M. A., Etherington D. J. The purification and properties of cathepsin L from rabbit liver. Biochem J. 1984 Jan 1;217(1):209–217. doi: 10.1042/bj2170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984 May;27(5):509–515. doi: 10.1002/art.1780270505. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Almond R. E., Etherington D. J. The immunohistochemical location of cathepsin L in rabbit skeletal muscle. Evidence for a fibre type dependent distribution. Histochemistry. 1987;86(4):379–383. doi: 10.1007/BF00494997. [DOI] [PubMed] [Google Scholar]
- Zuniga M. C., Hood L. E. Clonal variation in cell surface display of an H-2 protein lacking a cytoplasmic tail. J Cell Biol. 1986 Jan;102(1):1–10. doi: 10.1083/jcb.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]