Abstract
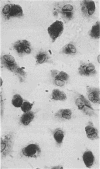
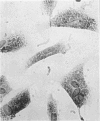
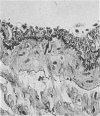
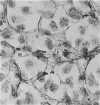
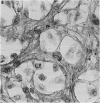
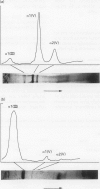
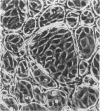
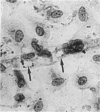
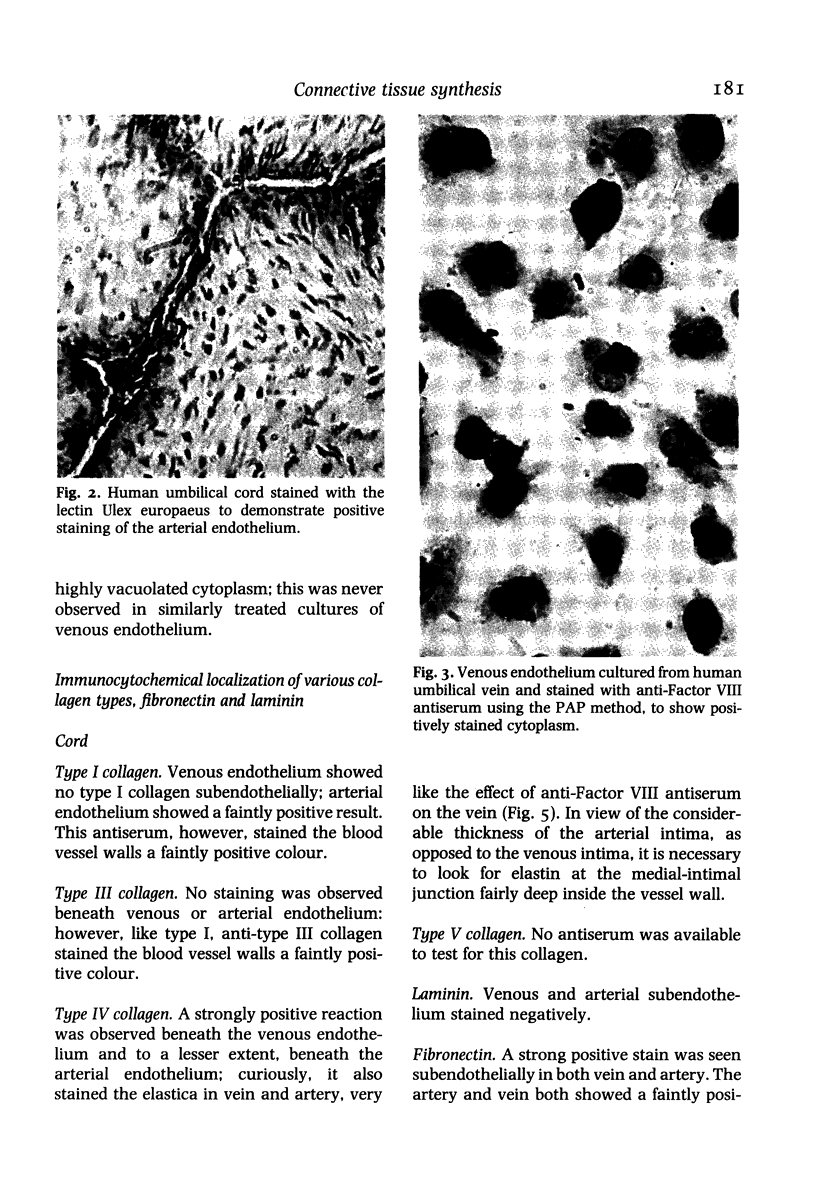
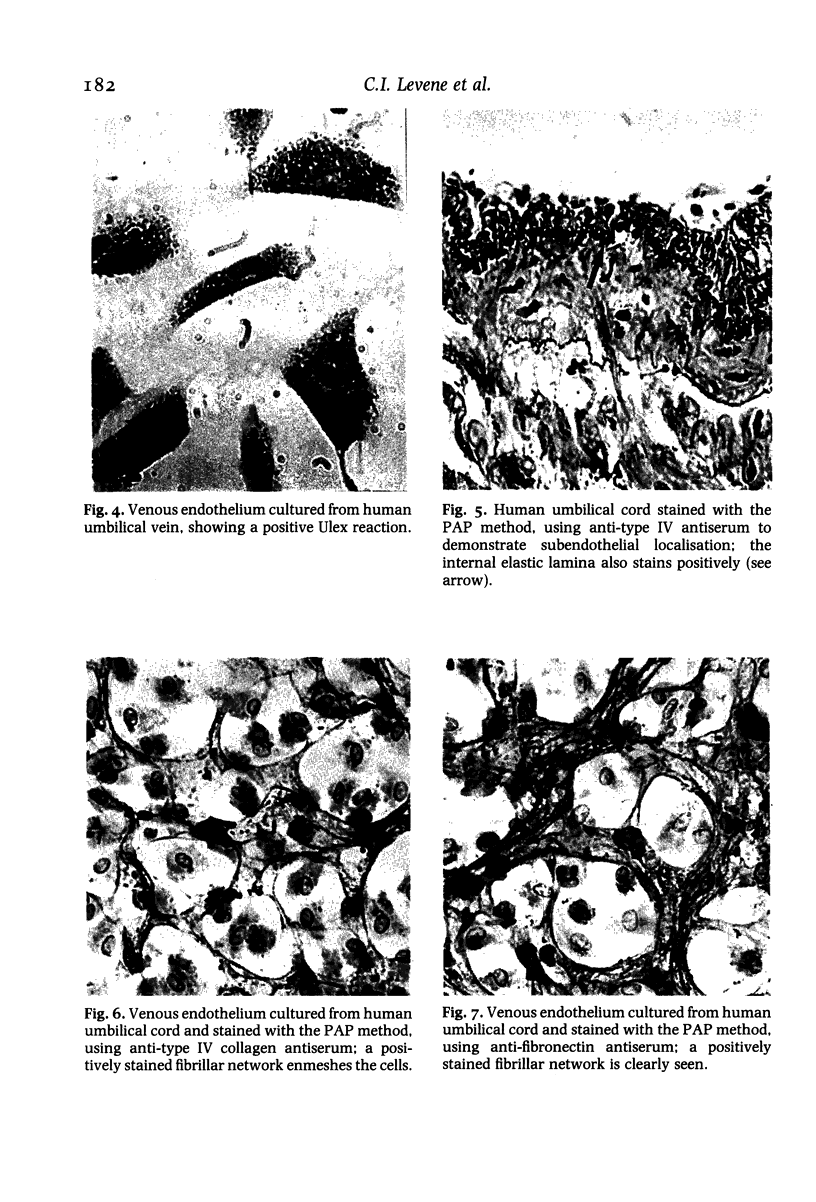
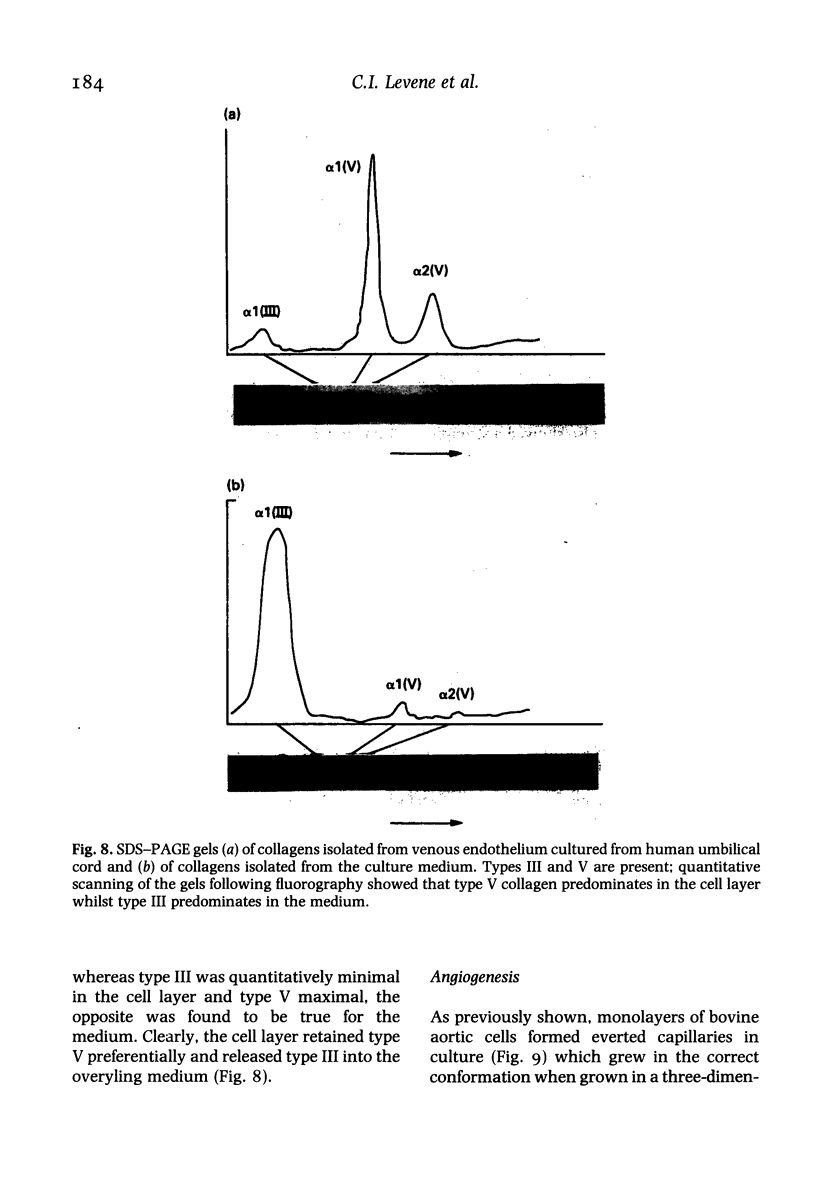
Immunocytochemistry has been used to identify endothelial cells in sections of human umbilical cord and in cultures of the venous and arterial endothelium, using Factor VIII and Ulex europaeus as endothelial markers. The connective tissue components, including various collagen types, fibronectin and laminin, were identified and localized in the cord and in both venous and arterial cultured endothelium. Interstitial collagens synthesized by the cultured cells were isolated and quantified. Angiogenic ability was examined. The effect of a noxious stimulus, 24 h hypoxia, was quantified in cultured venous endothelium. The results showed that cultured arterial endothelium possesses a vacuolated cytoplasm which is absent in venous endothelium. The major collagens observed in venous culture were types III and V; the latter was found mainly in the cell layer. Venous endothelium was angiogenic. It responded to hypoxia by producing fewer cells, more protein/10(6) cells but less collagen, both in absolute terms and as a percentage of protein/10(6) cells, thus behaving like cultured porcine and bovine aortic endothelium. Fibronectin was the major 'glue' associated with endothelium. We conclude that culture can reveal the synthetic potential of endothelium which the cord itself does not often show; moreover culture appears essential to demonstrate that arterial and venous endothelium behave differently from each other.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENE C. I., POOLE J. C. The collagen content of the normal and atherosclerotic human aortic intima. Br J Exp Pathol. 1962 Oct;43:469–471. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levene C. I., Bartlet C. P., Fornieri C., Heale G. Effect of hypoxia and carbon monoxide on collagen synthesis in cultured porcine and bovine aortic endothelium. Br J Exp Pathol. 1985 Aug;66(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- Levene C. I., Bartlet C., Heale G. Phenotypic changes in morphology and collagen polymorphism of cultured bovine and porcine aortic endothelium. Atherosclerosis. 1984 Jul;52(1):59–71. doi: 10.1016/0021-9150(84)90156-4. [DOI] [PubMed] [Google Scholar]
- Makarski J. S., Jr Evidence for reversible morphologic phenotypes (sprouts) of cultured endothelial cells. Cell Biol Int Rep. 1982 Mar;6(3):225–233. doi: 10.1016/0309-1651(82)90074-1. [DOI] [PubMed] [Google Scholar]