Abstract
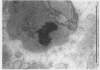
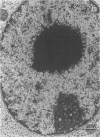
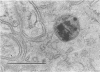
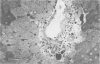
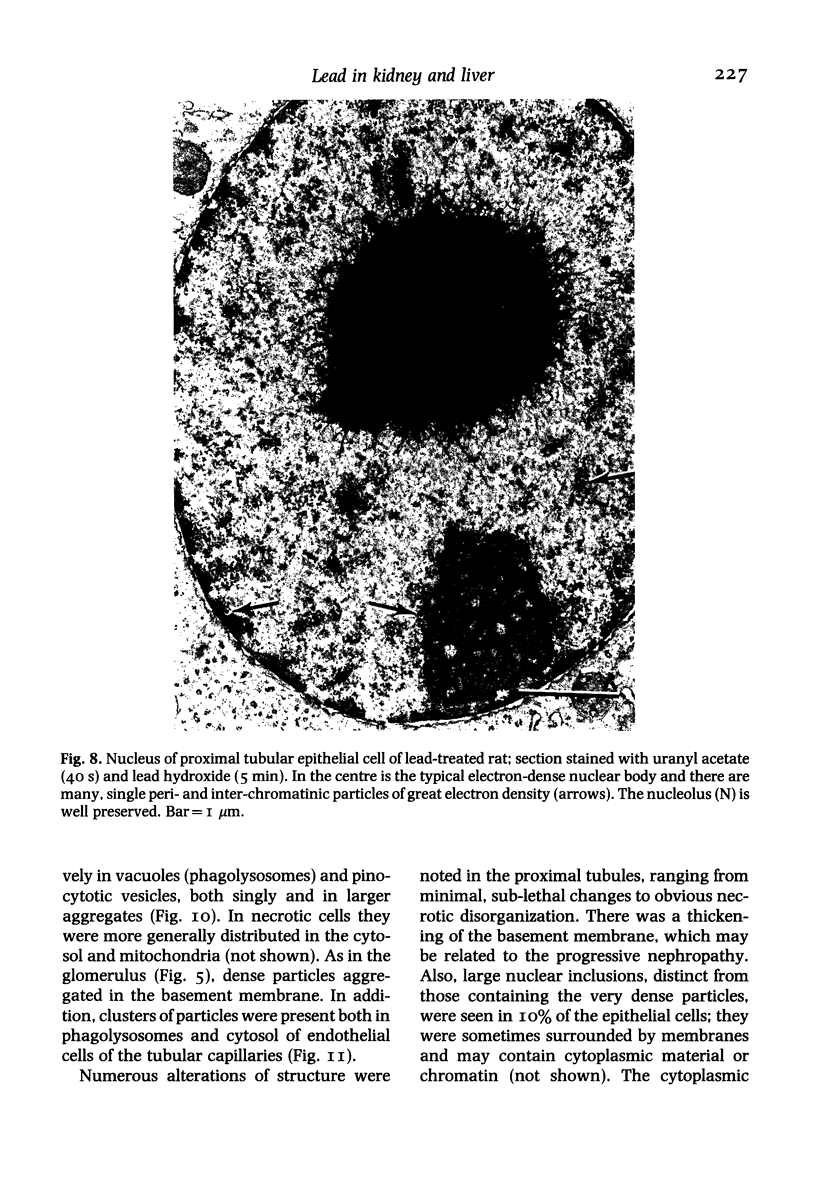
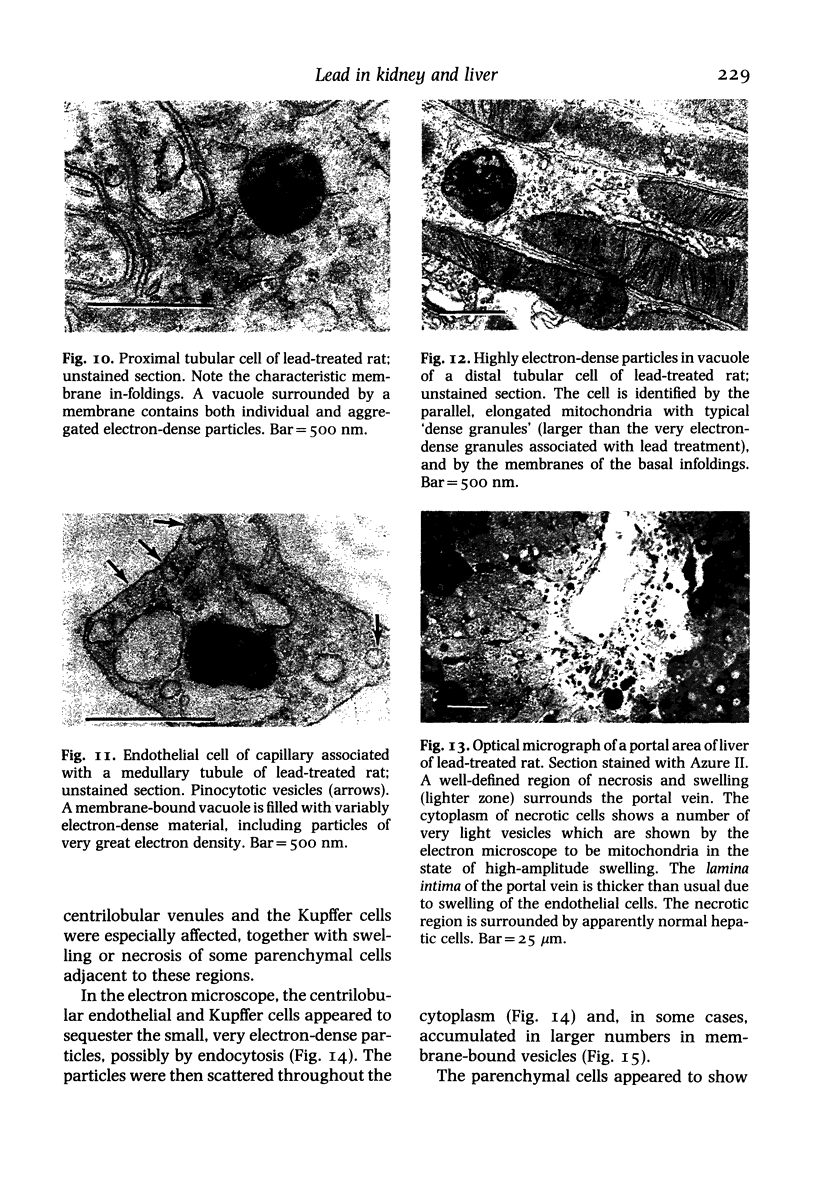
The subcellular distribution and ultrastructural effects of lead have been studied in the kidneys and liver of rats given lead acetate (600 ppm Pb2+) in their drinking water for 6 months. Control rats were given sodium acetate. Tissue samples were fixed in glutaraldehyde and examined by electron microscopy with and without staining. Unstained sections of both kidney and liver from lead-treated animals showed small particles (2-5 nm diameter) of very high electron density which appear to represent a deposited form of Pb2+. Ultrastructural changes in the kidneys were largely confined to glomeruli (swelling of endothelial cells, fusion of foot processes, thickening of basement membrane) and proximal tubules (ranging from minimal sub-lethal changes to necrotic disorganization). The electron-dense particles of Pb2+ occurred in large clusters in basement membranes. As individual particles, or small groups, they were numerous in nuclei of proximal epithelium but usually only a few, largely confined to vesicles or inclusion bodies, were present in the cytoplasm. Only when cells were markedly damaged morphologically were particles more generally distributed in the cytoplasm. Liver damage by Pb2+ was largely confined to centrilobular regions. Endothelial and Kupffer cells were the most affected; they often sequestered large numbers of the particles. In parenchymal cells, particles were few and mainly in vesicles, but they were more widely distributed in the cytoplasm when morphological injury was apparent. The free distribution of Pb2+ in liver and kidney seems to be limited by its deposition in basement membranes and sequestration in reticulo-endothelial cells; intracellular distribution in healthy cells is also limited, by deposition in nuclei (in kidney only) or cytoplasmic vesicles.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goyer R. A., Krall A., Kimball J. P. The renal tubule in lead poisoning. II. In vitro studies of mitochondrial structure and function. Lab Invest. 1968 Jul;19(1):78–83. [PubMed] [Google Scholar]
- Goyer R. A., Leonard D. L., Moore J. F., Rhyne B., Krigman M. R. Lead dosage and the role of the intranuclear inclusion body. An experimental study. Arch Environ Health. 1970 Jun;20(6):705–711. doi: 10.1080/00039896.1970.10665647. [DOI] [PubMed] [Google Scholar]
- Goyer R. A., Rhyne B. C. Pathological effects of lead. Int Rev Exp Pathol. 1973;12:1–77. [PubMed] [Google Scholar]
- Macadam R. F. The early glomerular lesion in human and rabbit lead poisoning. Br J Exp Pathol. 1969 Jun;50(3):239–240. [PMC free article] [PubMed] [Google Scholar]
- Port C. D., Baxter D. W., Richter W. R. The Mongolian gerbil as a model for lead toxicity. I. Studies of acute poisoning. Am J Pathol. 1974 Jul;76(1):79–94. [PMC free article] [PubMed] [Google Scholar]
- Russo M. A., van Rossum G. D., Galeotti T. Observations on the regulation of cell volume and metabolic control in vitro; changes in the composition and ultrastructure of liver slices under conditions of varying metabolic and transporting activity. J Membr Biol. 1977 Mar 8;31(3):267–299. doi: 10.1007/BF01869409. [DOI] [PubMed] [Google Scholar]
- Vostál J., Heller J. Renal excretory mechanisms of heavy metals. I. Transtubular transport of heavy metal ions in the avian kidney. Environ Res. 1968 Sep;2(1):1–10. doi: 10.1016/0013-9351(68)90001-7. [DOI] [PubMed] [Google Scholar]
- van Rossum G. D., Kapoor S. C., Rabinowitz M. S. Effects of inorganic lead in vitro on ion exchanges and respiratory metabolism of rat kidney cortex. Arch Toxicol. 1985 Jan;56(3):175–181. doi: 10.1007/BF00333423. [DOI] [PubMed] [Google Scholar]