Abstract
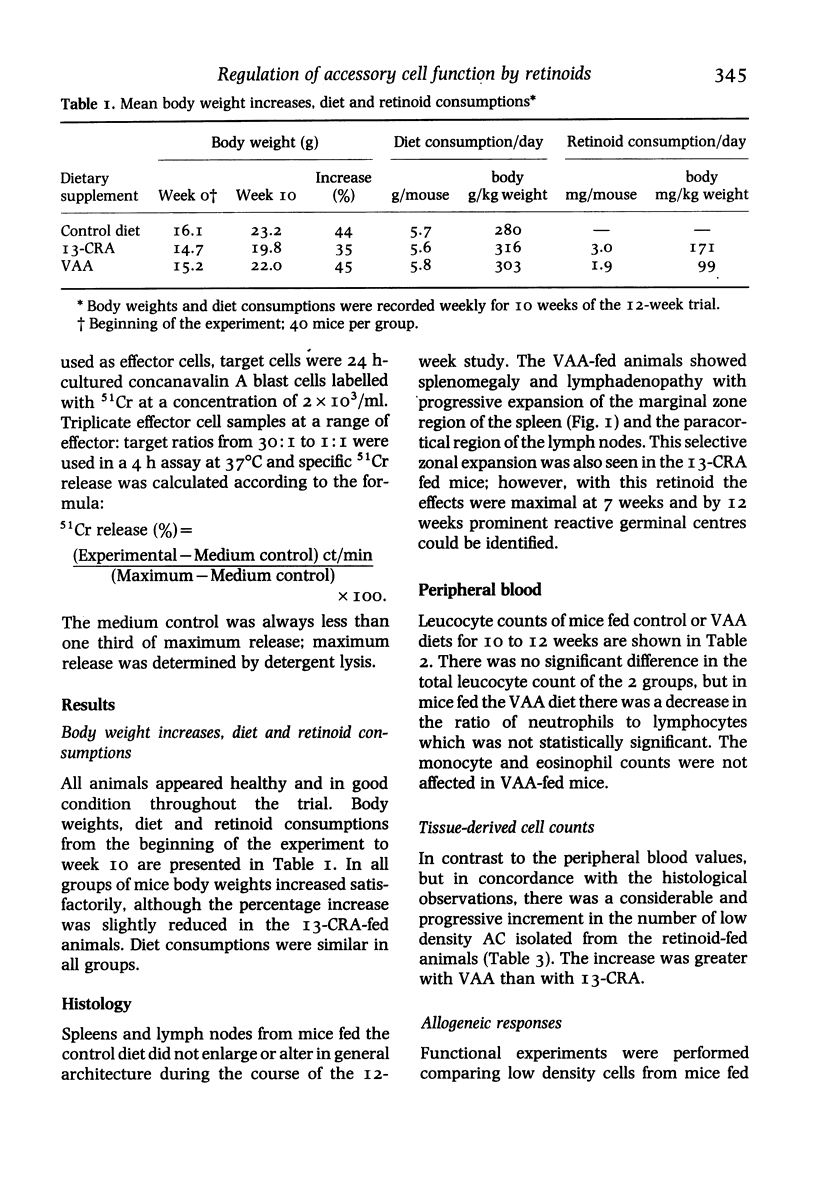
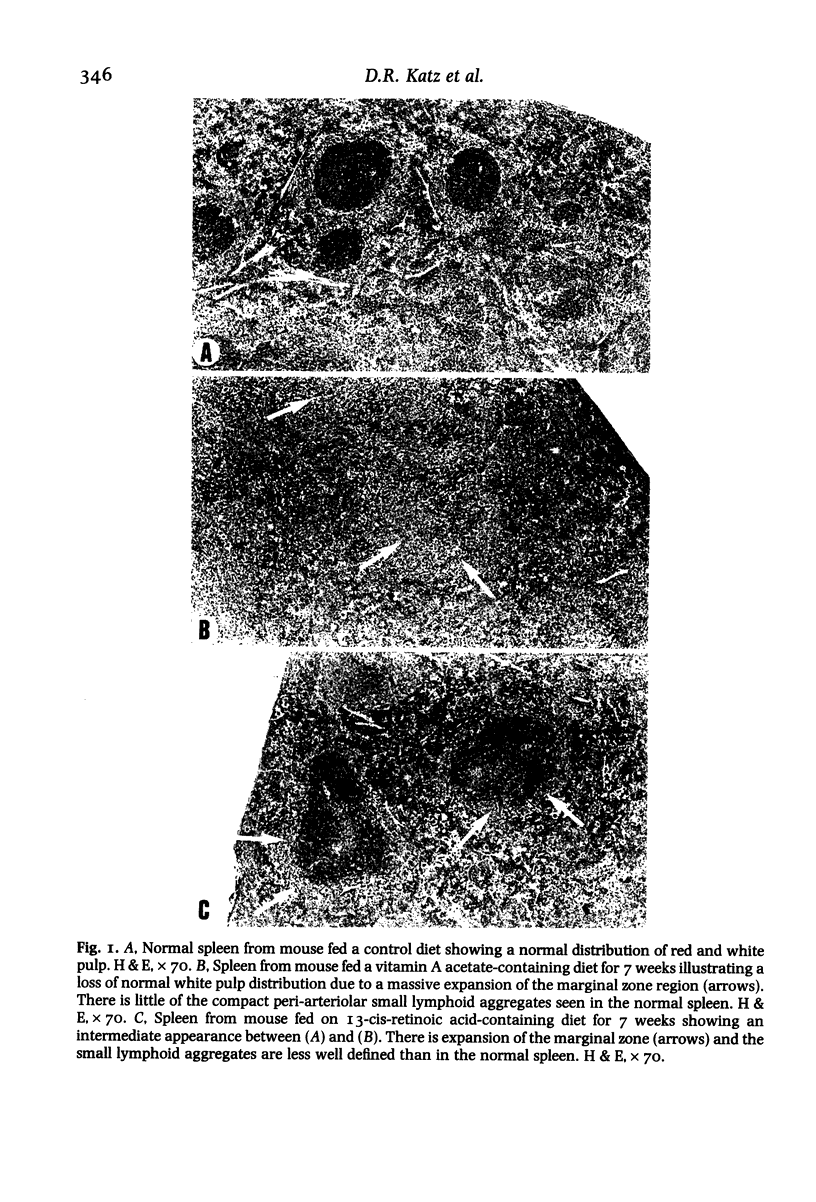
This study examines the effects of in-vivo immune regulation by vitamin A acetate (VAA) and 13-cis-retinoic acid (13-CRA) on in-vitro accessory cell function. Mice were fed a control diet, or diet containing VAA or 13-CRA, and monitored by body weight gains and diet consumptions at weekly intervals. At 4, 7 and 12 weeks mice were killed, differential blood counts performed and accessory cells isolated from lymphomedullary tissues. Histology confirmed that the chief feature of the lymphomedullary organs of the VAA-fed animals was an expansion of the splenic marginal zone and the paracortical region of the lymph nodes. There was an increase in the number of accessory cells present, and this included both dendritic cells and macrophages. The accessory cell function of these cells was also increased, as evidenced by both alloproliferative and allocytotoxic responses in vitro. In 13-CRA-fed animals the effects were similar to those seen with VAA, but were less pronounced. We suggest that the primary effects of these compounds on in-vivo immunoregulation could be due to their promotion of accessory cell function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dennert G., Crowley C., Kouba J., Lotan R. Retinoic acid stimulation of the induction of mouse killer T-cells in allogeneic and syngeneic systems. J Natl Cancer Inst. 1979 Jan;62(1):89–94. [PubMed] [Google Scholar]
- Fossum S., Ford W. L. The organization of cell populations within lymph nodes: their origin, life history and functional relationships. Histopathology. 1985 May;9(5):469–499. doi: 10.1111/j.1365-2559.1985.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Lotan R., Neumann G., Deutsch V. Identification and characterization of specific changes induced by retinoic acid in cell surface glycoconjugates of S91 murine melanoma cells. Cancer Res. 1983 Jan;43(1):303–312. [PubMed] [Google Scholar]
- Malkovský M., Edwards A. J., Hunt R., Palmer L., Medawar P. B. T-cell-mediated enhancement of host-versus-graft reactivity in mice fed a diet enriched in vitamin A acetate. Nature. 1983 Mar 24;302(5906):338–340. doi: 10.1038/302338a0. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Medawar P. B., Thatcher D. R., Toy J., Hunt R., Rayfield L. S., Doré C. Acquired immunological tolerance of foreign cells is impaired by recombinant interleukin 2 or vitamin A acetate. Proc Natl Acad Sci U S A. 1985 Jan;82(2):536–538. doi: 10.1073/pnas.82.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Medawar P., Hunt R., Palmer L., Doré C. A diet enriched in vitamin A acetate or in vivo administration of interleukin-2 can counteract a tolerogenic stimulus. Proc R Soc Lond B Biol Sci. 1984 Feb 22;220(1221):439–445. doi: 10.1098/rspb.1984.0012. [DOI] [PubMed] [Google Scholar]
- Medawar P. B., Hunt R. Anti-cancer action of retinoids. Immunology. 1981 Feb;42(2):349–353. [PMC free article] [PubMed] [Google Scholar]
- Patek P. Q., Collins J. L., Yogeeswaran G., Dennert G. Anti-tumor potential of retinoic acid: stimulation of immune mediated effectors. Int J Cancer. 1979 Nov 15;24(5):624–628. doi: 10.1002/ijc.2910240516. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Allison A. C. Mode of action of adjuvants: retinol and other lysosome-labilizing agents as adjuvants. J Immunol. 1970 Jan;104(1):119–127. [PubMed] [Google Scholar]




