Abstract
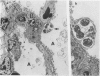
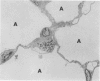
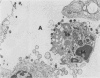
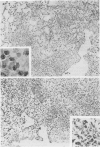
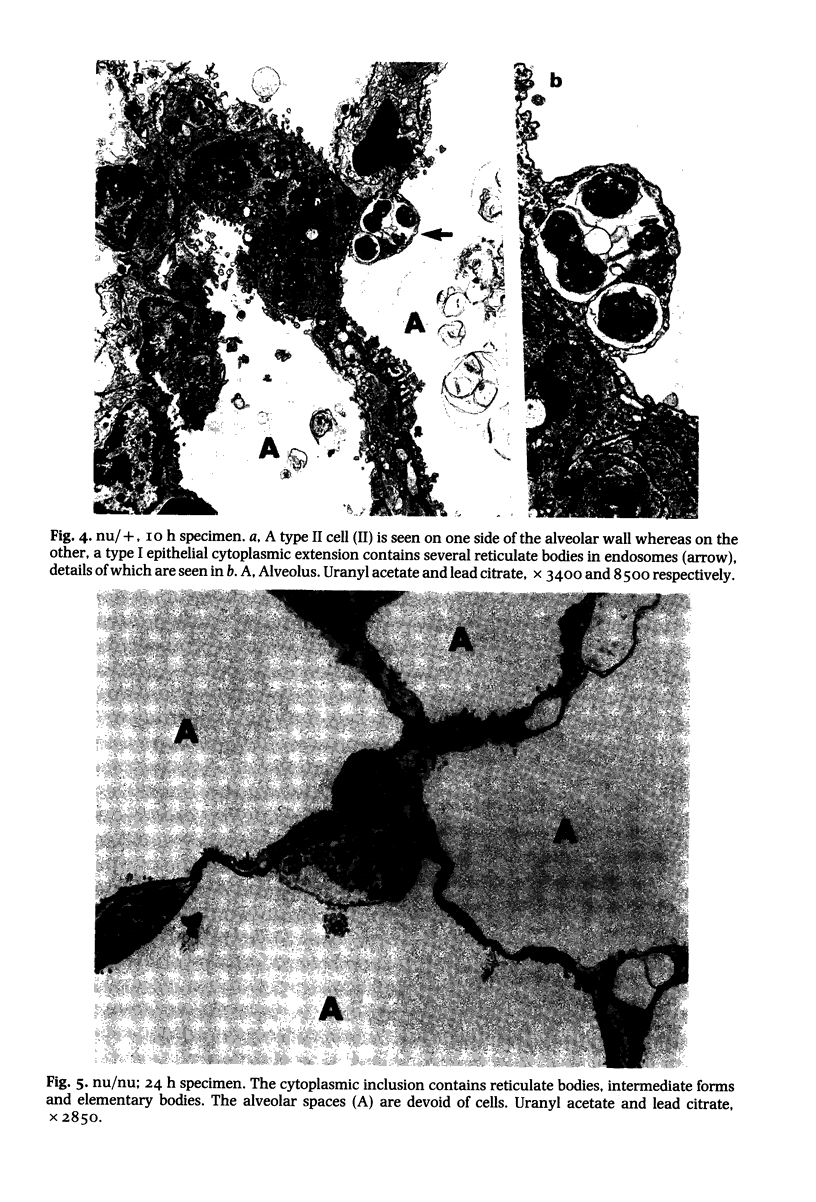
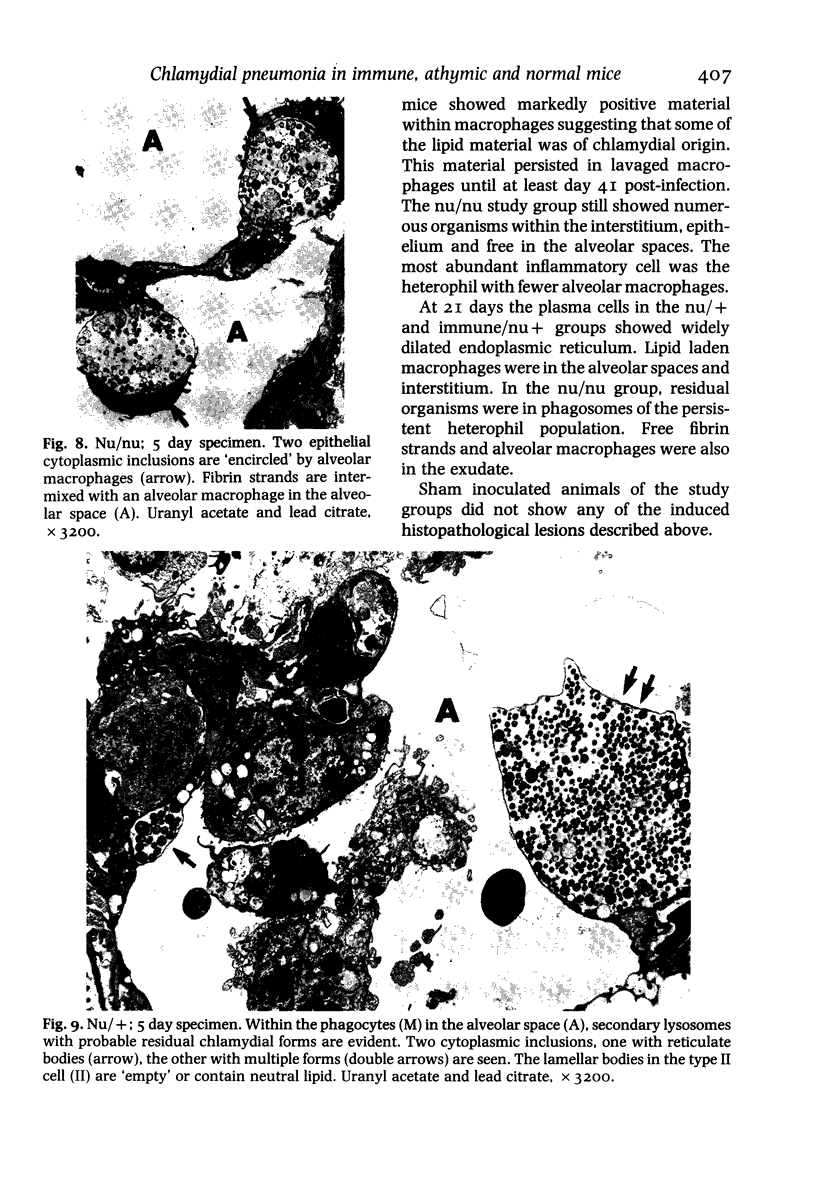
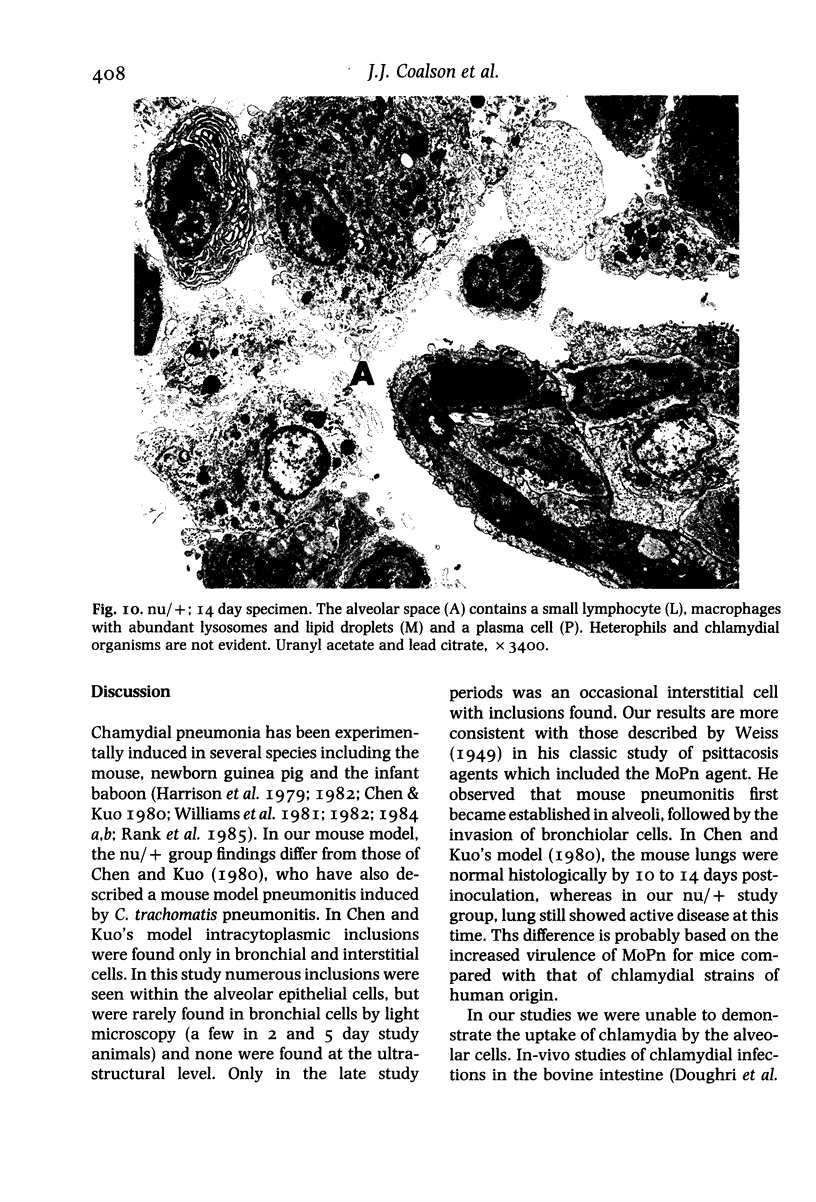
This paper compares the histopathology of pneumonia due to murine Chlamydia trachomatis (MoPn, mouse pneumonitis agent) in susceptible athymic nude mice (nu/nu), resistant heterozygous littermates (nu/+) and very resistant immunized nu/+ mice. While all groups had an early heterophil response, successful host defence correlated with the presence of large numbers of plasma cells, lymphocytes, monocytes, and lipid laden macrophages. Reticulate bodies were seen in all groups, predominantly in type I alveolar epithelial cells. By 24 h in the immune nu/+ group, no intact organisms were visible. Optimal control of infection was thus rapid and not clearly related to heterophils. These studies show that the histopathology of chlamydial infection may be quite atypical in the immunocompromised host, mononuclear cells seem critical in host defence, and B cell activation with plasma cell infiltration is dependent on intact T cell function in this model.
Full text
PDF


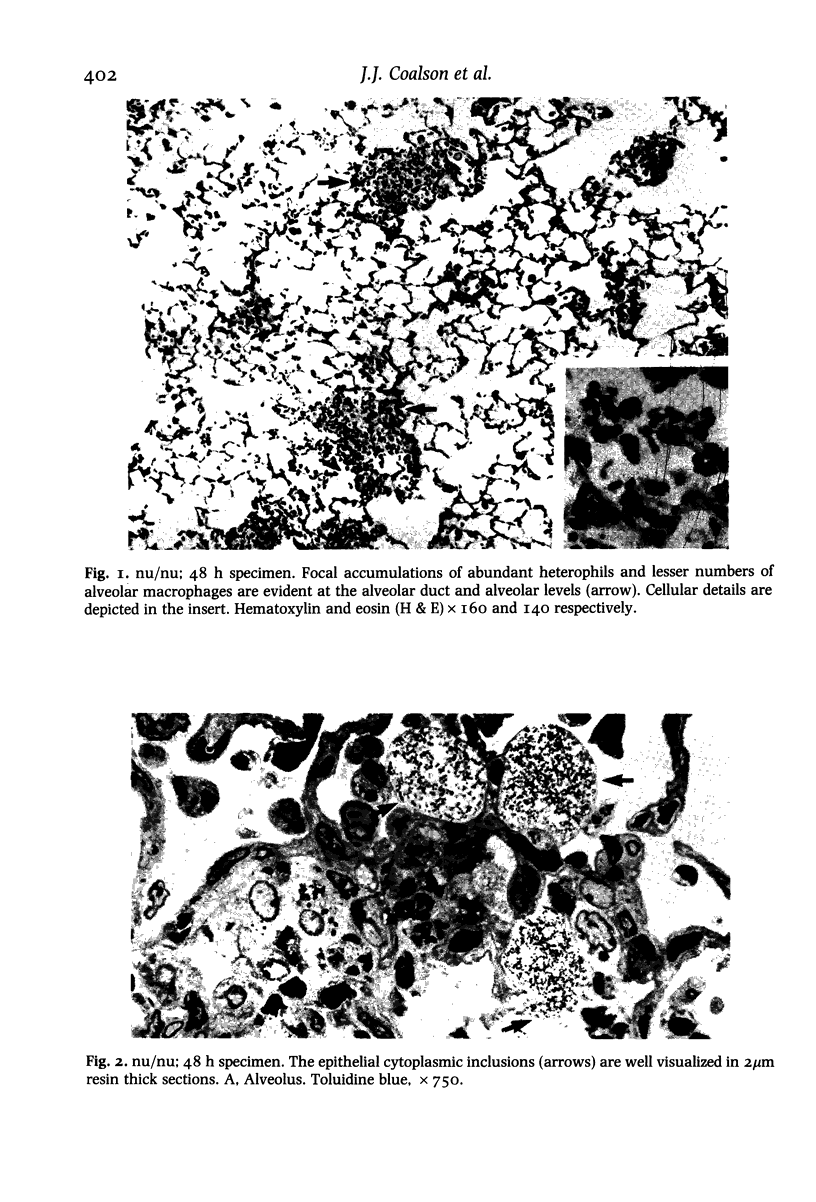

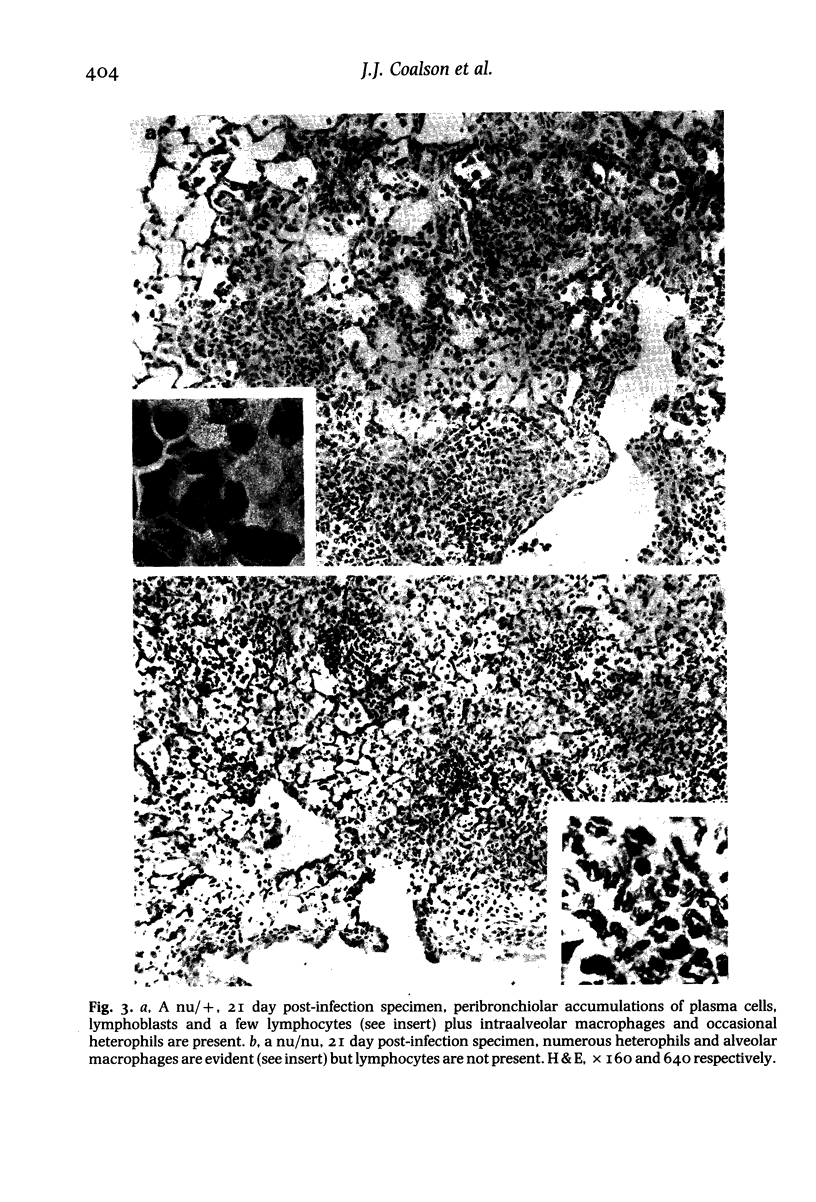




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acute hepatitis B associated with gynaecological surgery. Lancet. 1980 Jan 5;1(8158):1–6. [PubMed] [Google Scholar]
- Bard J., Levitt D. Chlamydia trachomatis stimulates human peripheral blood B lymphocytes to proliferate and secrete polyclonal immunoglobulins in vitro. Infect Immun. 1984 Jan;43(1):84–92. doi: 10.1128/iai.43.1.84-92.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Kuo C. A mouse model of pneumonitis induced by Chlamydia trachomatis: morphologic, microbiologic, and immunologic studies. Am J Pathol. 1980 Aug;100(2):365–382. [PMC free article] [PubMed] [Google Scholar]
- Doughri A. M., Altera K. P., Storz J. Host cell range of chlamydial infection in the neonatal bovine gut. J Comp Pathol. 1973 Jan;83(1):107–114. doi: 10.1016/0021-9975(73)90033-9. [DOI] [PubMed] [Google Scholar]
- Doughri A. M., Storz J., Altera K. P. Mode of entry and release of chlamydiae in infections of intestinal epithelial cells. J Infect Dis. 1972 Dec;126(6):652–657. doi: 10.1093/infdis/126.6.652. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Alexander E. R., Chiang W. T., Giddens W. E., Jr, Boyce J. T., Benjamin D., Gale J. L. Experimental nasopharyngitis and pneumonia caused by Chlamydia trachomatis in infant baboons: histopathologic comparison with a case in a human infant. J Infect Dis. 1979 Feb;139(2):141–146. doi: 10.1093/infdis/139.2.141. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Lee S. M., Lucas D. O. Chlamydia trachomatis pneumonitis in the C57BL/KsJ mouse: pathologic and immunologic features. J Lab Clin Med. 1982 Dec;100(6):953–962. [PubMed] [Google Scholar]
- Ito J. I., Jr, Comess K. A., Alexander E. R., Harrison H. R., Ray C. G., Kiviat J., Sobonya R. E. Pneumonia due to chlamydia trachomatis in an immunocompromised adult. N Engl J Med. 1982 Jul 8;307(2):95–98. doi: 10.1056/NEJM198207083070205. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Rank R. G., Hough A. J., Jr, Jacobs R. F., Cohen C., Barron A. L. Chlamydial pneumonitis induced in newborn guinea pigs. Infect Immun. 1985 Apr;48(1):153–158. doi: 10.1128/iai.48.1.153-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B., Sumaya C. V. The role of antibody in host defense against the agent of mouse pneumonitis. J Infect Dis. 1982 Feb;145(2):200–205. doi: 10.1093/infdis/145.2.200. [DOI] [PubMed] [Google Scholar]