Abstract
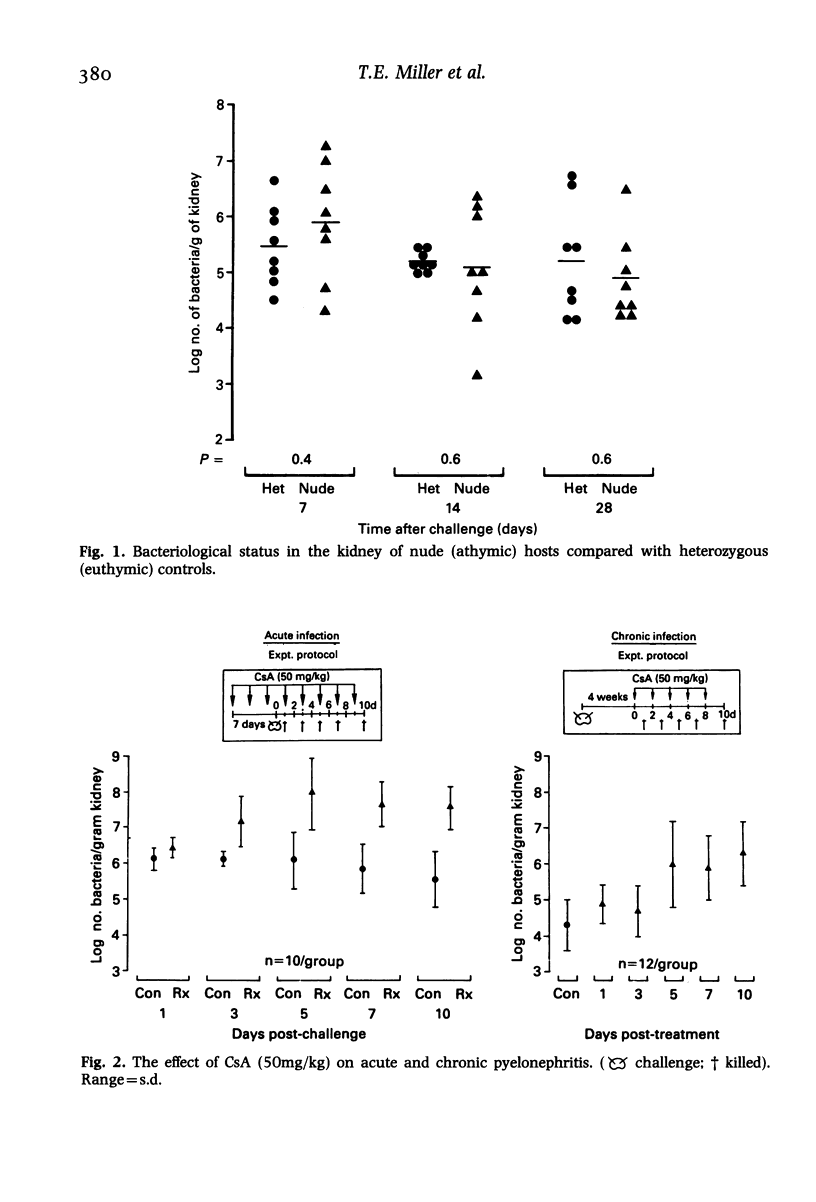
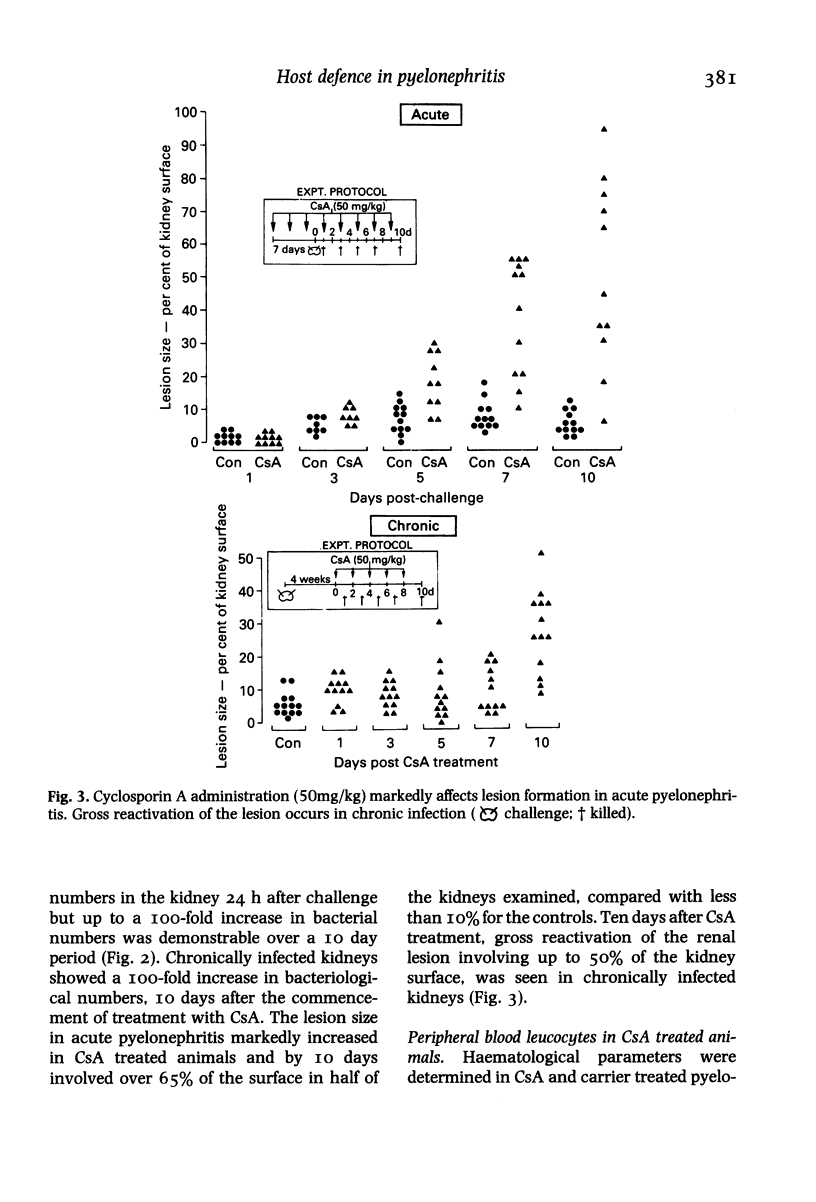
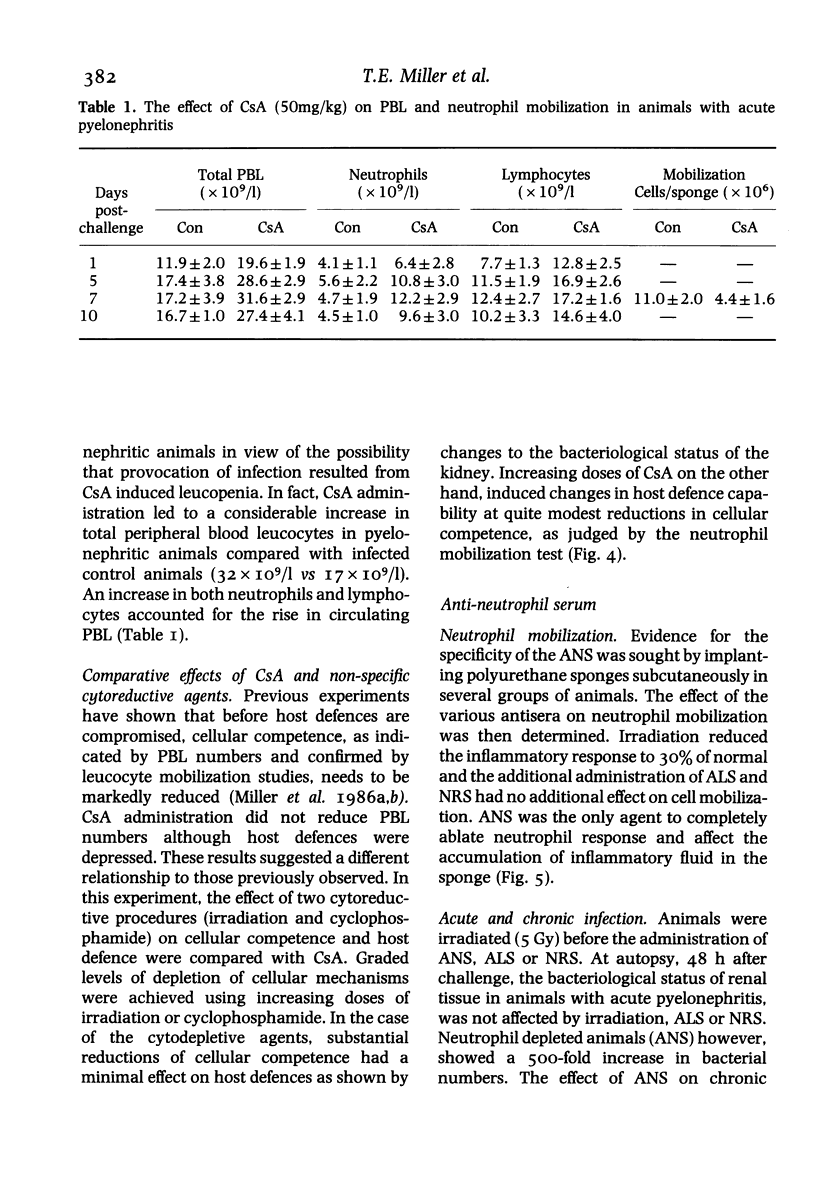
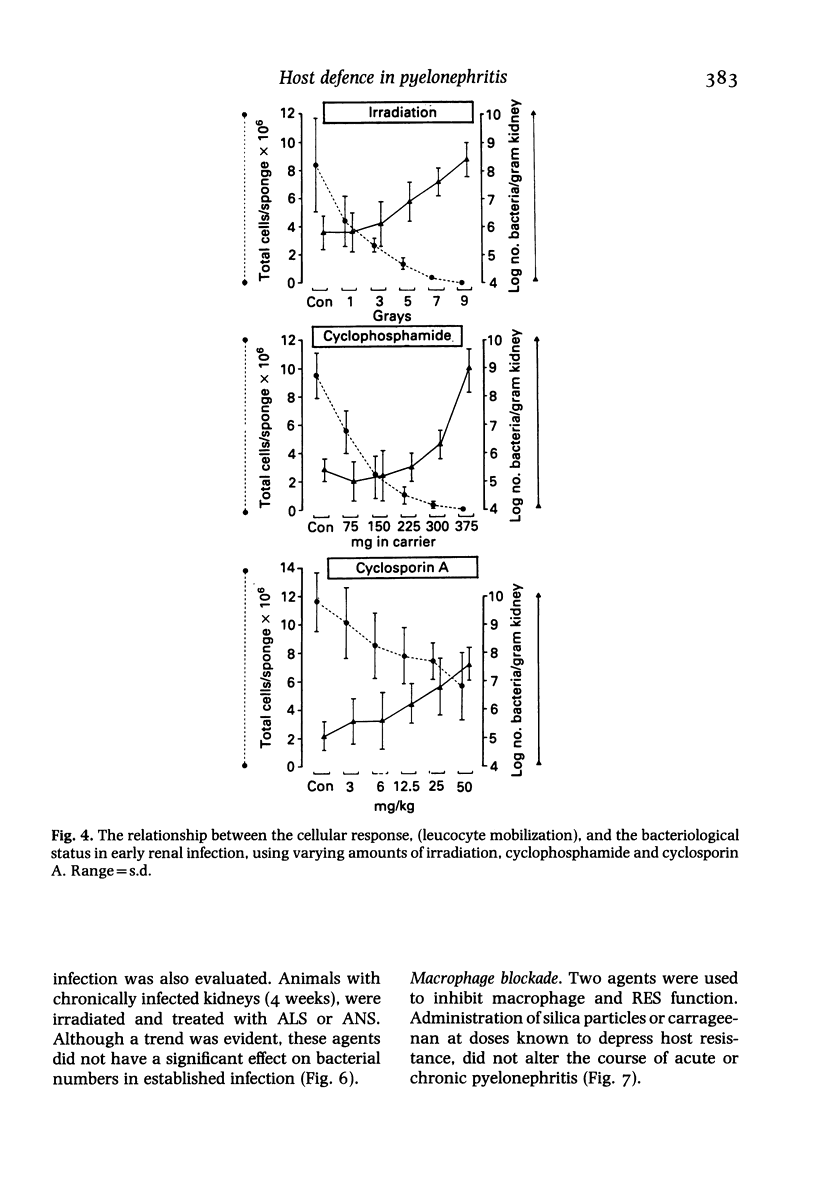
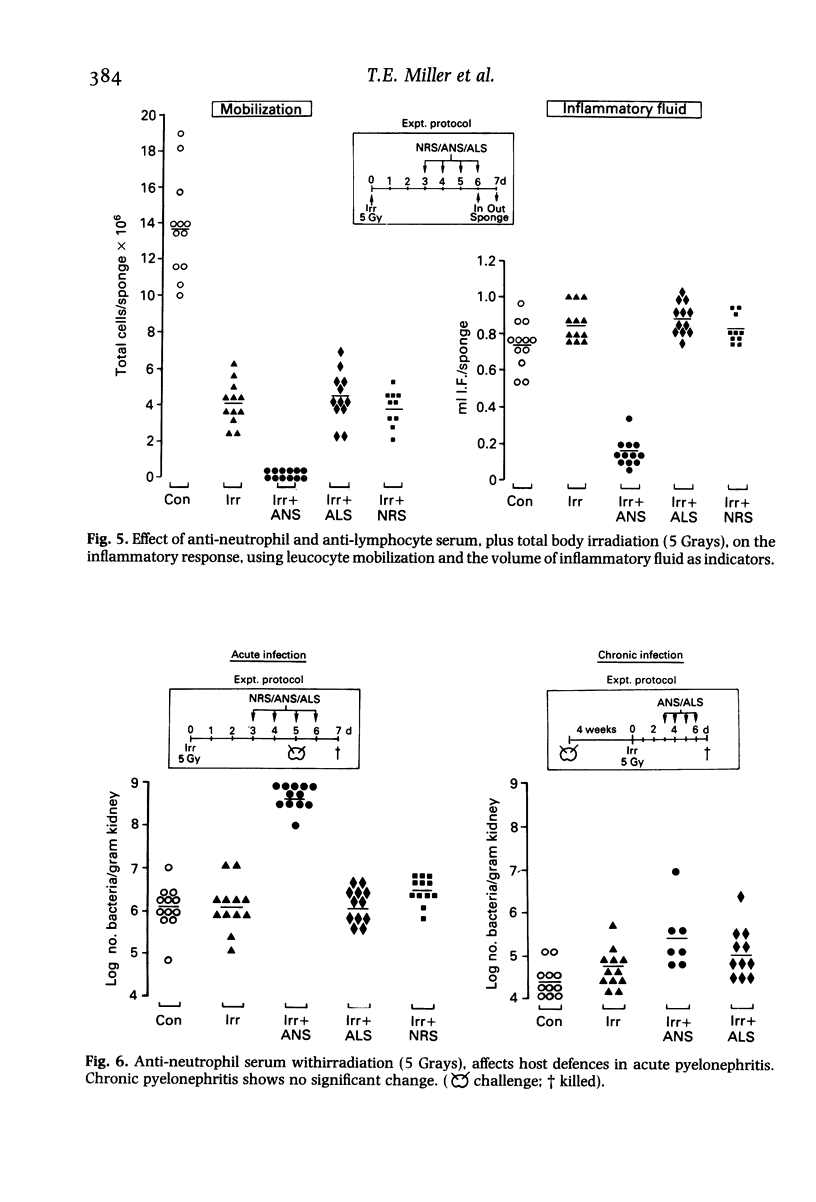
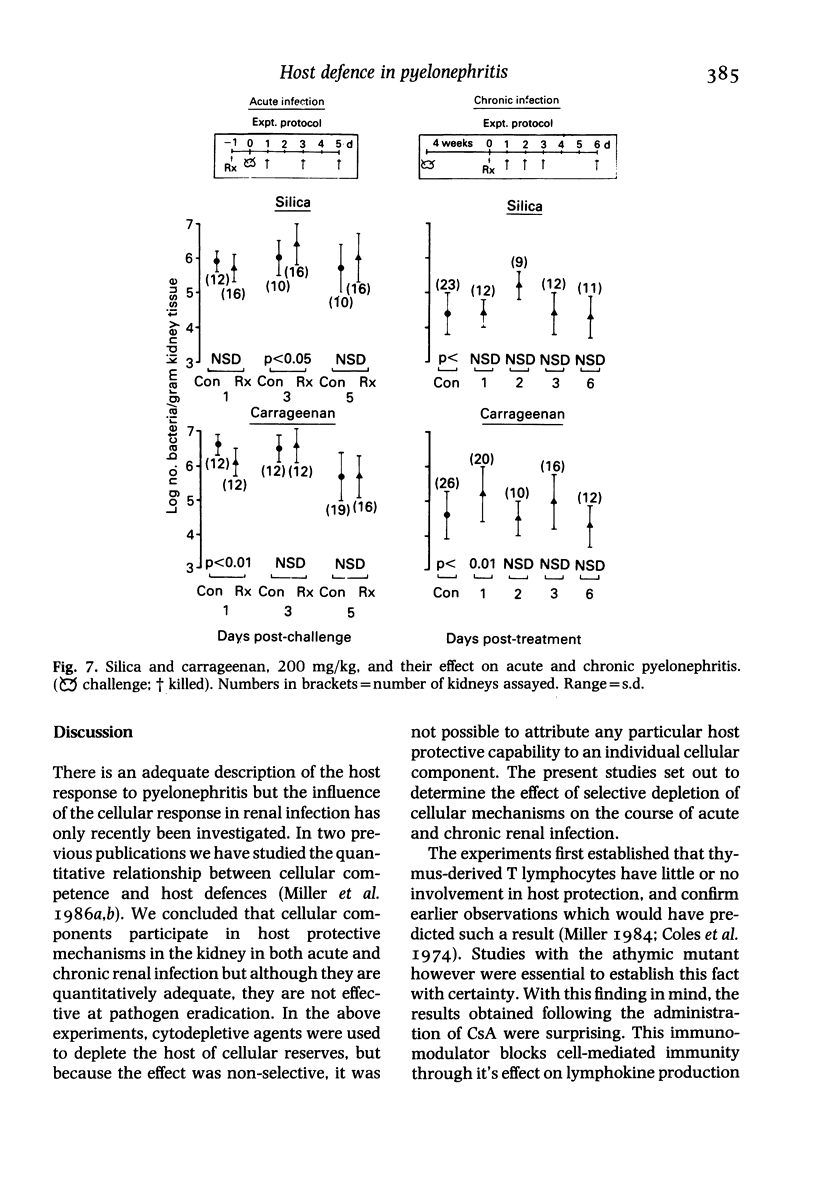
Hosts were depleted of individual cellular components to determine the effects of these manipulations on cellular defence mechanisms in acute and chronic pyelonephritis. T-lymphocytes were found to have little or no involvement in host protection but cyclosporin A administration had a dramatic effect on the gross pathology and bacteriological status of experimentally induced pyelonephritis. This change represented a major depression of host defence status. Cyclosporin A also activated resolved lesions in chronic pyelonephritis, associated with an increase in bacterial numbers. Administration of antineutrophil serum also led to a 1000-fold increase in bacterial numbers in the acute phase but had little effect on the host-parasite balance in chronic pyelonephritis. Macrophage blockade, on the other hand, did not affect the course of either acute or chronic infection. These studies have provided additional information on the immunobiology of experimental pyelonephritis and have focussed attention on the role of neutrophils, and an unidentified mechanism, affected by cyclosporin A, in host defence to renal infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coles G. A., Chick S., Hopkins M., Ling R., Radford N. J. The role of the T cell in experimental pyelonephritis. Clin Exp Immunol. 1974 Apr;16(4):629–636. [PMC free article] [PubMed] [Google Scholar]
- Ernst J. D., Decazes J. M., Sande M. A. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect Immun. 1983 Jul;41(1):275–279. doi: 10.1128/iai.41.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Kongshavn P. A. Effect of silica on resistance of mice to Entamoeba histolytica infection. Infect Immun. 1984 Aug;45(2):399–402. doi: 10.1128/iai.45.2.399-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J. M., Arroyo O., Chaves F., Lomonte B., Cerdas L. Pathogenesis of myonecrosis induced by coral snake (Micrurus nigrocinctus) venom in mice. Br J Exp Pathol. 1986 Feb;67(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. Effects of intravenous silica on immune and non-immune functions of the murine host. J Immunol. 1975 Jul;115(1):41–48. [PubMed] [Google Scholar]
- Löliger C., Lehmann-Grube F. Mechanism of recovery from acute virus infection. II. Effect of treatment of mice with cyclosporin A on their ability to eliminate the lymphocytic choriomeningitis virus. Med Microbiol Immunol. 1985;174(4):187–196. doi: 10.1007/BF02123695. [DOI] [PubMed] [Google Scholar]
- Meddens M. J., Thompson J., Eulderink F., Bauer W. C., Mattie H., van Furth R. Role of granulocytes in experimental Streptococcus sanguis endocarditis. Infect Immun. 1982 Apr;36(1):325–332. doi: 10.1128/iai.36.1.325-332.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Findon G., Cawley S., Clarke I. Cellular basis of host defence in pyelonephritis. II. Acute infection. Br J Exp Pathol. 1986 Apr;67(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Miller T. Pyelonephritis: the role of cell-mediated immunity defined in a congenitally athymic rat. Kidney Int. 1984 Dec;26(6):816–822. doi: 10.1038/ki.1984.223. [DOI] [PubMed] [Google Scholar]
- Moffat F. L., Falk R. E., Teodorczyk-Injeyan J., Clark A. G., Gilas T., Falk M., Dalfen R., Rotstein L. E., McDonell M., Makowka L. Reversal of cyclosporine-induced mortality with a synthetic polymeric immunostimulant in a murine model of fecal peritonitis. Transplantation. 1985 Apr;39(4):369–374. doi: 10.1097/00007890-198504000-00006. [DOI] [PubMed] [Google Scholar]
- Monga D. P. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. 1981 Jun;32(3):975–978. doi: 10.1128/iai.32.3.975-978.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormrod D. J., Cawley S., Miller T. E. Extended immunosuppression with cyclophosphamide using controlled-release polymeric implants. Int J Immunopharmacol. 1985;7(4):443–448. doi: 10.1016/0192-0561(85)90062-1. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985 Oct;50(1):22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Takeya K., Shimotori S., Taniguchi T., Nomoto K. Cellular mechanisms in the protection against infection by Listeria monocytogenes in mice. J Gen Microbiol. 1977 Jun;100(2):373–379. doi: 10.1099/00221287-100-2-373. [DOI] [PubMed] [Google Scholar]



