Abstract
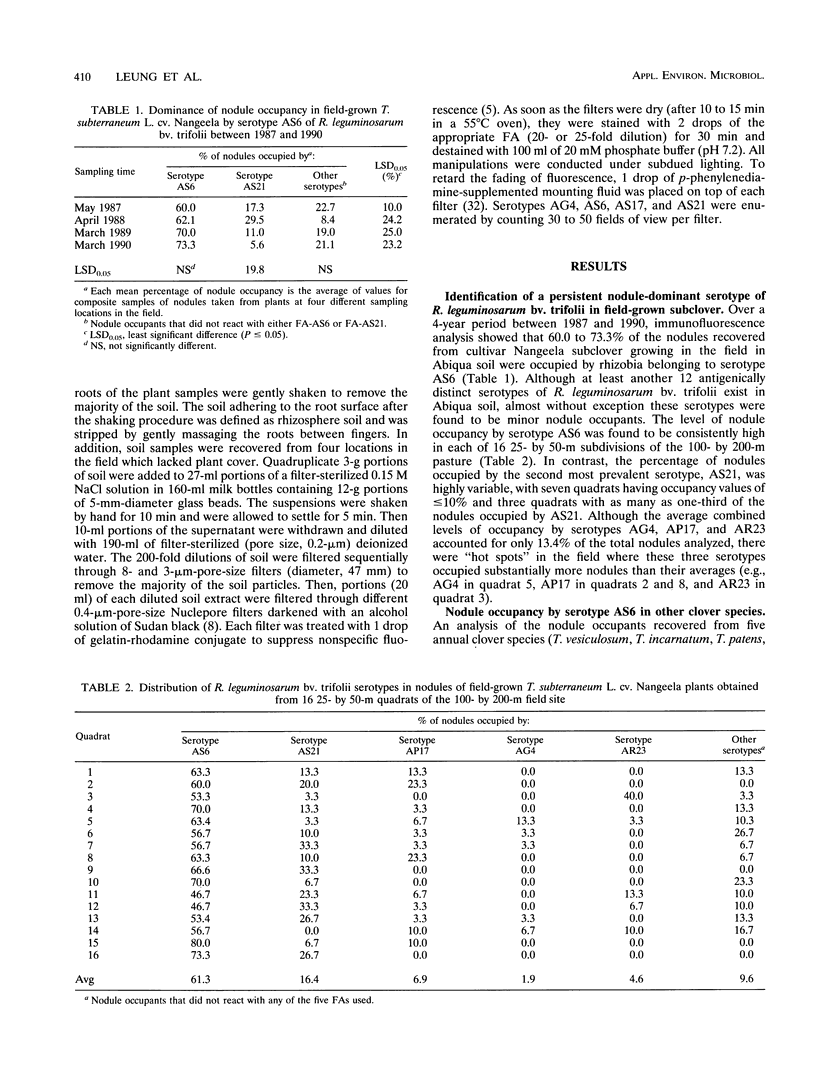
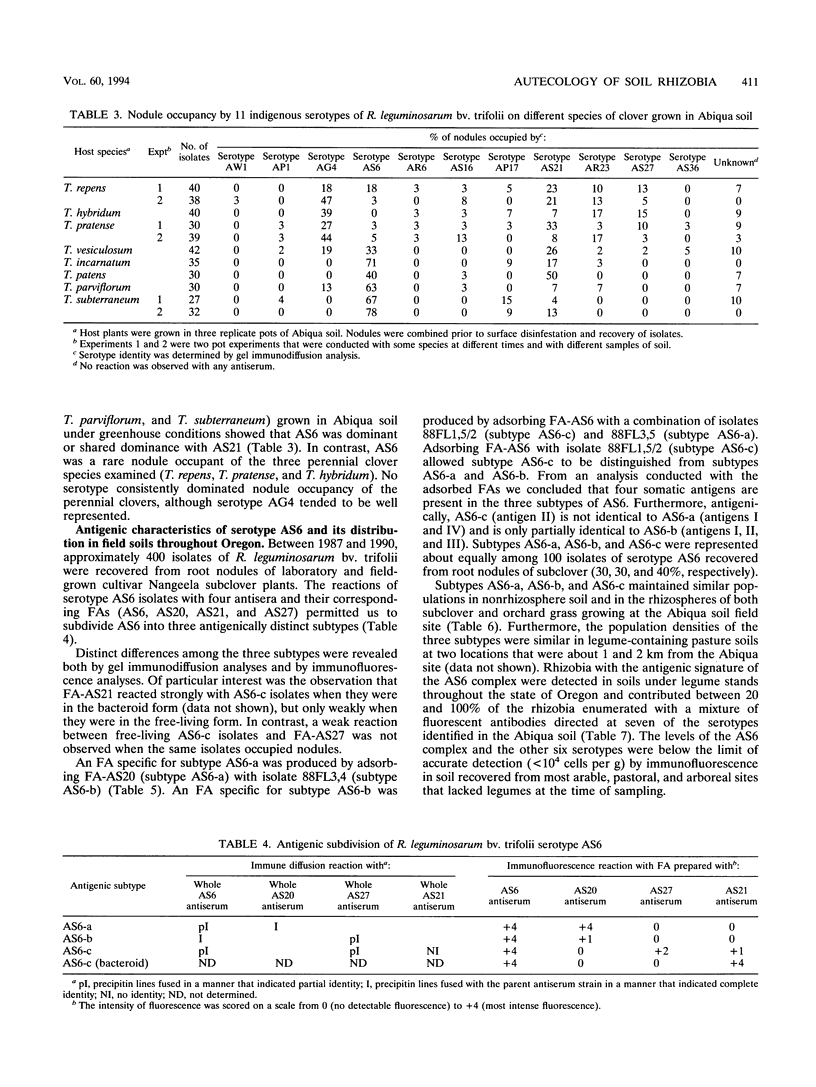
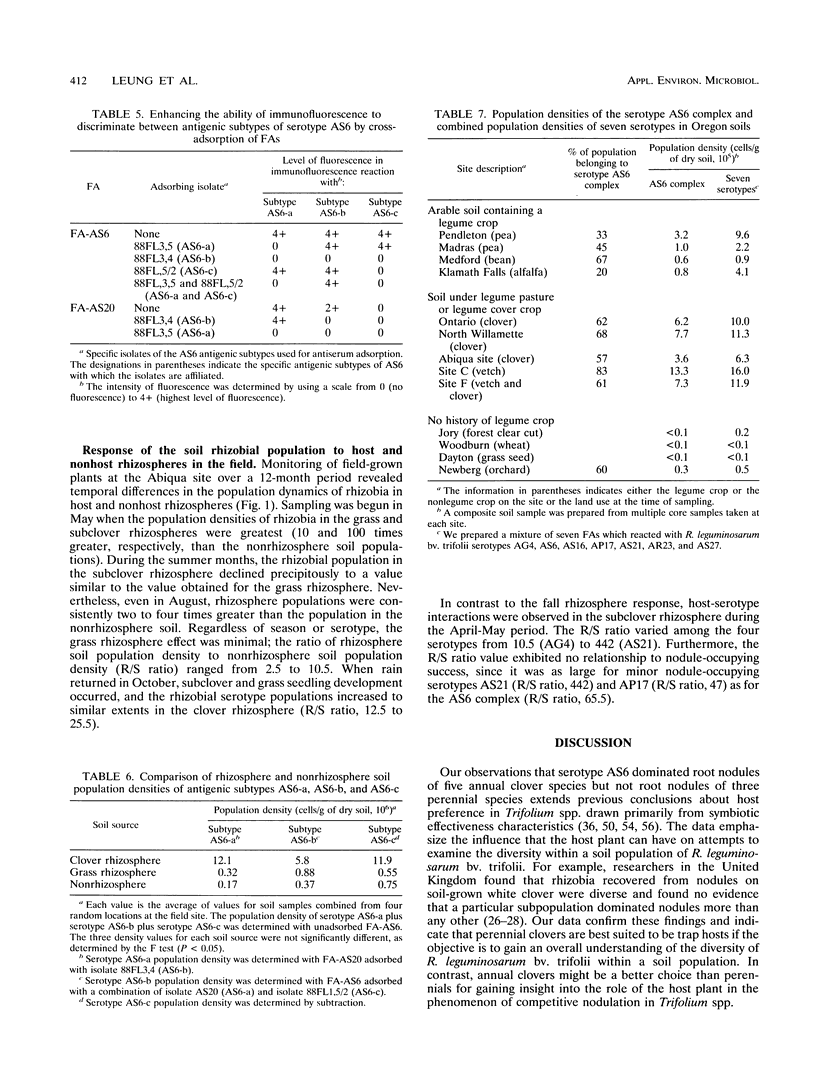
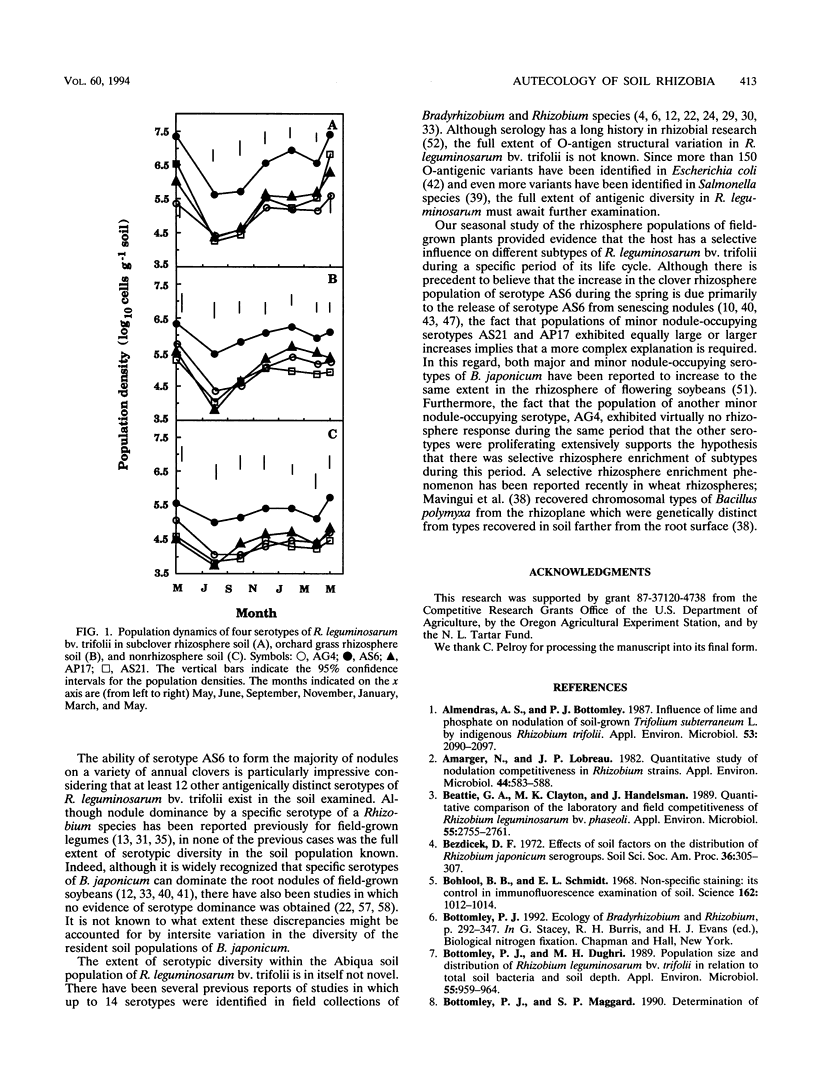
Although at least 13 antigenically distinct serotypes of Rhizobium leguminosarum bv. trifolii exist in an Abiqua silty clay loam soil, one serotype, AS6, occupies ≥50% of the root nodules formed on field-grown subclover and between 33 and 78% of the nodules formed on five annual clover species grown in the same soil under laboratory conditions. The dominance of subclover nodules by serotype AS6 was reproducible over a 4-year sampling period and throughout the entire 200- by 100-m pasture examined. Serotype AS6 was composed of three antigenically distinct subtypes (AS6-a, AS6-b, and AS6-c). Each subtype contributed about one-third of the AS6 isolates recovered from nodules of field-grown subclover plants and maintained similar population densities in nonrhizosphere and rhizosphere soil. Rhizobia with the AS6 antigenic signature accounted for from 20 to 100% of the soil populations of R. leguminosarum in arable and pasture soils under legumes throughout the state of Oregon. Over a 12-month period, the population densities of the serotype AS6 complex and three minor nodule-occupying serotypes (AG4, AP17, and AS21) were measured in the rhizospheres of field-grown subclover and orchard grass and in nonrhizosphere Abiqua soil. Regardless of season or serotype, the orchard grass rhizosphere effect was minimal, with the ratio between rhizosphere and nonrhizosphere serotype population densities ranging between 2.5 (midsummer) and 10.5 (spring). In contrast, the magnitude of the subclover rhizosphere effect varied seasonally and among serotypes. Between October and December the ratios for all serotypes were similar (12.5 to 25.5). However, in the spring (April and May), the magnitude of the rhizosphere effect varied among the indigenous serotypes (ratios, 10.5 to 442) and for minor nodule-occupying serotypes AS21 (ratio, 442) and AP17 (ratio, 47) was as great as, or even greater than, the magnitude of the rhizosphere effect observed with the AS6 complex (ratio, 65.5).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendras A. S., Bottomley P. J. Influence of Lime and Phosphate on Nodulation of Soil-Grown Trifolium subterraneum L. by Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2090–2097. doi: 10.1128/aem.53.9.2090-2097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie G. A., Clayton M. K., Handelsman J. Quantitative comparison of the laboratory and field competitiveness of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1989 Nov;55(11):2755–2761. doi: 10.1128/aem.55.11.2755-2761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Bottomley P. J., Dughri M. H. Population Size and Distribution of Rhizobium leguminosarum bv. trifolii in Relation to Total Soil Bacteria and Soil Depth. Appl Environ Microbiol. 1989 Apr;55(4):959–964. doi: 10.1128/aem.55.4.959-964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Autecology in Rhizospheres and Nodulating Behavior of Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1014–1019. doi: 10.1128/aem.52.5.1014-1019.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Interstrain Competition between Representatives of Indigenous Serotypes of Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1020–1025. doi: 10.1128/aem.52.5.1020-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling D. N., Broughton W. J. Competition for nodulation of legumes. Annu Rev Microbiol. 1986;40:131–157. doi: 10.1146/annurev.mi.40.100186.001023. [DOI] [PubMed] [Google Scholar]
- George M. L., Robert F. M. Competition among Rhizobium leguminosarum bv. phaseoli strains for nodulation of common bean. Can J Microbiol. 1992 Feb;38(2):157–160. doi: 10.1139/m92-026. [DOI] [PubMed] [Google Scholar]
- Holland A. A. Serologic characteristics of certain root-nodule bacteria of legumes. Antonie Van Leeuwenhoek. 1966;32(4):410–418. doi: 10.1007/BF02097492. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Lochner H. H., Strijdom B. W., Law I. J. Unaltered Nodulation Competitiveness of a Strain of Bradyrhizobium sp. (Lotus) after a Decade in Soil. Appl Environ Microbiol. 1989 Nov;55(11):3000–3008. doi: 10.1128/aem.55.11.3000-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavingui P., Laguerre G., Berge O., Heulin T. Genetic and Phenotypic Diversity of Bacillus polymyxa in Soil and in the Wheat Rhizosphere. Appl Environ Microbiol. 1992 Jun;58(6):1894–1903. doi: 10.1128/aem.58.6.1894-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Sadowsky M. J. Genetics of competition for nodulation of legumes. Annu Rev Microbiol. 1992;46:399–428. doi: 10.1146/annurev.mi.46.100192.002151. [DOI] [PubMed] [Google Scholar]



