Abstract
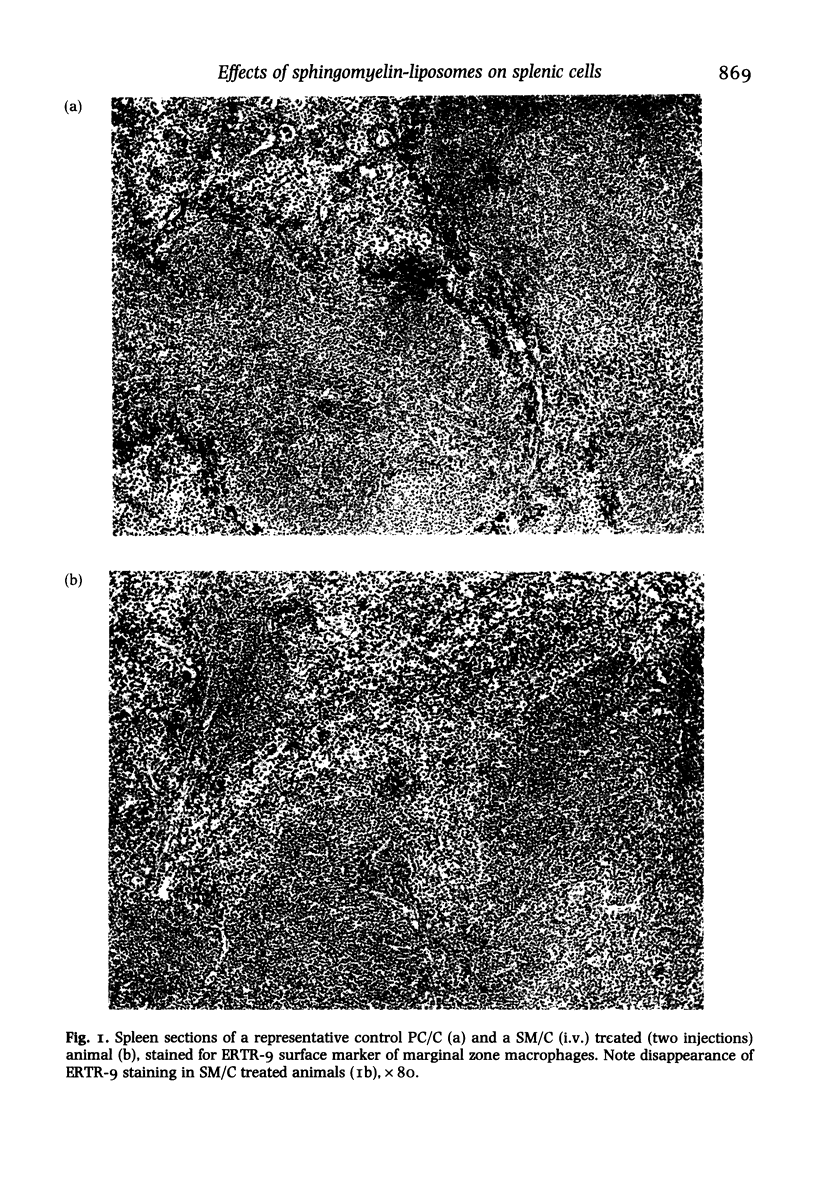
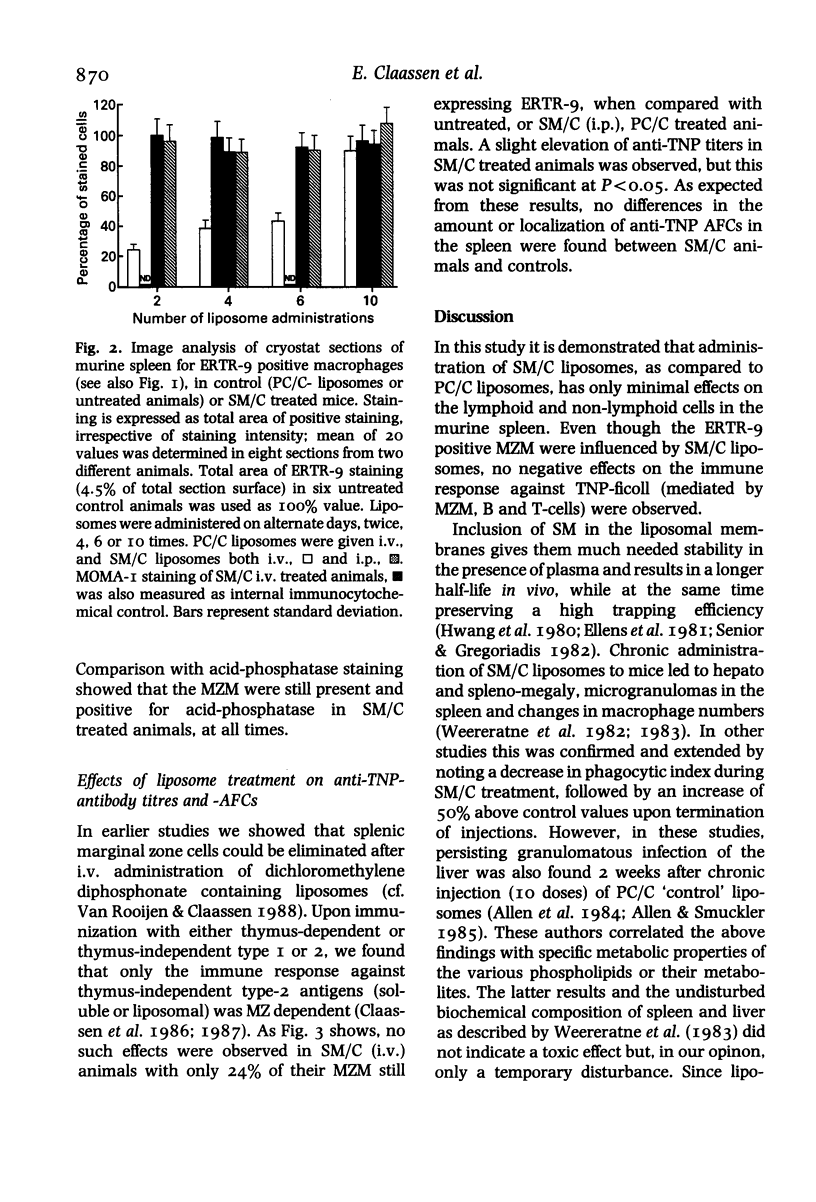
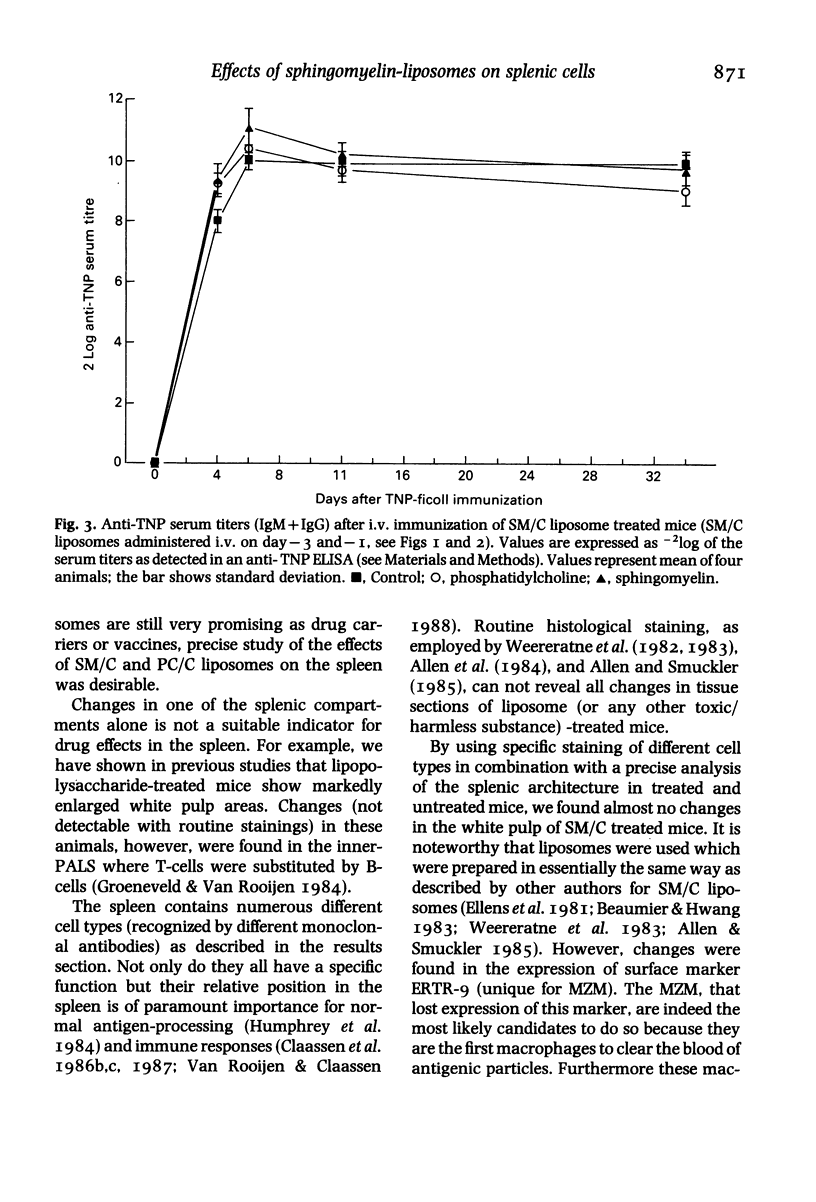
Mice were injected with sphingomyelin/cholesterol or phosphatidylcholine/cholesterol (PC/C) liposomes, from twice up to 10 times, on alternate days. Administration of sphingomyelin/cholesterol (SM/C) liposomes gave rise to hepato and splenomegaly, microgranulomatous infections and changes in macrophage numbers and activity in spleen and liver. Enzyme and immuno-cytochemical methods were used, to demonstrate the effect of liposomes on the lymphoid and non-lymphoid cell populations, on cryostat sections of the spleen. Routine histological staining, of sphingomyelin/cholesterol treated animals, showed no drastic changes in morphology or compartmentalization of the spleen, apart from a small enlargement (with some microgranulomas) of the red pulp. No significant differences were found in the presence or localization of T-helper, T-cytotoxic/suppressor, T-total-lymphocytes, B-total-lymphocytes, red pulp macrophages, marginal metallophils, or non-lymphoid dendritic cells. However, a transient suppression of cells expressing marginal zone macrophage surface marker ERTR-9, was observed between the second and eighth (intravenous) administration of sphingomyelin/cholesterol liposomes. Immunization of these animals with trinitrophenyl (TNP)-ficoll, a thymus-independent type-2 antigen which is specifically processed by marginal zone macrophages (MZM), showed that these cells were not suppressed with regard to their immunological function. We conclude that chronic administration of sphingomyelin liposomes influences macrophages, probably through a general phagocytic-system overload, but not permanent or damaging changes in splenic cell populations or immunological functions occur.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Murray L., MacKeigan S., Shah M. Chronic liposome administration in mice: effects on reticuloendothelial function and tissue distribution. J Pharmacol Exp Ther. 1984 Apr;229(1):267–275. [PubMed] [Google Scholar]
- Allen T. M., Smuckler E. A. Liver pathology accompanying chronic liposome administration in mouse. Res Commun Chem Pathol Pharmacol. 1985 Nov;50(2):281–290. [PubMed] [Google Scholar]
- Amlot P. L., Grennan D., Humphrey J. H. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985 May;15(5):508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- Baum B. J., McDonald J. A., Crystal R. G. Metabolic fate of the major cell surface protein of normal human fibroblasts. Biochem Biophys Res Commun. 1977 Nov 7;79(1):8–15. doi: 10.1016/0006-291x(77)90053-5. [DOI] [PubMed] [Google Scholar]
- Beaumier P. L., Hwang K. J. Effects of liposome size on the degradation of bovine brain sphingomyelin/cholesterol liposomes in the mouse liver. Biochim Biophys Acta. 1983 May 26;731(1):23–30. doi: 10.1016/0005-2736(83)90393-0. [DOI] [PubMed] [Google Scholar]
- Braun J., Unanue E. R. B lymphocyte biology studied with anti-Ig antibodies. Immunol Rev. 1980;52:3–28. doi: 10.1111/j.1600-065x.1980.tb00328.x. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., van Rooijen N. Immunomodulation with liposomes: the immune response elicited by liposomes with entrapped dichloromethylene-diphosphonate and surface-associated antigen or hapten. Immunology. 1987 Apr;60(4):509–515. [PMC free article] [PubMed] [Google Scholar]
- Classen E., Van Rooijen N. Preparation and characteristics of dichloromethylene diphosphonate-containing liposomes. J Microencapsul. 1986 Apr-Jun;3(2):109–114. doi: 10.3109/02652048609031565. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P., Dijkstra C. D., Boorsma D. M., van Rooijen N. Characterization of lymphoid and nonlymphoid cells in the white pulp of the spleen using immunohistoperoxidase techniques and enzyme-histochemistry. Experientia. 1985 Feb 15;41(2):209–215. doi: 10.1007/BF02002615. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Gray D., Chassoux D., MacLennan I. C., Bazin H. Selective depression of thymus-independent anti-DNP antibody responses induced by adult but not neonatal splenectomy. Clin Exp Immunol. 1985 Apr;60(1):78–86. [PMC free article] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N. In vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Reduction of T-lymphocytes and phagocytosis in the inner parts of the periarteriolar lymphocyte sheath. Cell Tissue Res. 1984;236(3):637–642. doi: 10.1007/BF00217233. [DOI] [PubMed] [Google Scholar]
- Grover A., Sundharadas G. Effect of liposomes on lymphocytes: induction of proliferation of B lymphocytes and potentiation of the cytotoxic response of T lymphocytes to alloantigens. Eur J Immunol. 1986 Jun;16(6):665–670. doi: 10.1002/eji.1830160613. [DOI] [PubMed] [Google Scholar]
- Hwang K. J., Luk K. F., Beaumier P. L. Hepatic uptake and degradation of unilamellar sphingomyelin/cholesterol liposomes: a kinetic study. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4030–4034. doi: 10.1073/pnas.77.7.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumararatne D. S., Bazin H., MacLennan I. C. Marginal zones: the major B cell compartment of rat spleens. Eur J Immunol. 1981 Nov;11(11):858–864. doi: 10.1002/eji.1830111103. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Mongini P. K., Sieckmann D., Paul W. E. Role of T lymphocytes in the response to TNP-AECM-Ficoll. J Immunol. 1980 Sep;125(3):1066–1070. [PubMed] [Google Scholar]
- Ng M. H., Ng W. S., Ho W. K., Fung K. P., Lamelin J. P. Modulation of phytohemagglutinin-mediated lymphocyte stimulation by egg lecithin. Exp Cell Res. 1978 Oct 15;116(2):387–395. doi: 10.1016/0014-4827(78)90462-7. [DOI] [PubMed] [Google Scholar]
- Nuzzo F., Sala F., Biondi O., Casati A., Cestaro B., de Carli L. Liposomes induce chromosome aberrations in human cultured cells. Exp Cell Res. 1985 Apr;157(2):397–408. doi: 10.1016/0014-4827(85)90125-9. [DOI] [PubMed] [Google Scholar]
- Rivnay B., Globerson A., Shinitzky M. Peturbation of lymphocyte response to concanavalin A by exogenous cholesterol and lecithin. Eur J Immunol. 1978 Mar;8(3):185–189. doi: 10.1002/eji.1830080309. [DOI] [PubMed] [Google Scholar]
- Senior J., Gregoriadis G. Is half-life of circulating liposomes determined by changes in their permeability? FEBS Lett. 1982 Aug 16;145(1):109–114. doi: 10.1016/0014-5793(82)81216-7. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Veerman A. J., van Ewijk W. White pulp compartments in the spleen of rats and mice. A light and electron microscopic study of lymphoid and non-lymphoid celltypes in T- and B-areas. Cell Tissue Res. 1975;156(4):417–441. doi: 10.1007/BF00225103. [DOI] [PubMed] [Google Scholar]
- Weereratne E. A., Gregoriadis G., Crow J. Toxicity of sphingomyelin-containing liposomes after chronic injection into mice. Br J Exp Pathol. 1983 Dec;64(6):670–676. [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Nieuwenhuis P. Compartments, domains and migration pathways of lymphoid cells in the splenic pulp. Experientia. 1985 Feb 15;41(2):199–208. doi: 10.1007/BF02002614. [DOI] [PubMed] [Google Scholar]
- van Vliet E., Melis M., van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985 Jan;33(1):40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]



