Abstract
Statistically significant declines in chronic disability prevalence rates were observed in the elderly United States population between the 1982 and 1989 National Long Term Care Surveys (NLTCS). The 1994 NLTCS was used to investigate whether disability rate declines continued to 1994. The 1982, 1984, 1989, and 1994 NLTCS employ the same sample design and instrumentation so that trends in disability can be estimated with minimal sampling and measurement bias. Age (5-year categories from 65 to >95)-specific rates were calculated for the 1982 NLTCS and applied to United States Census Bureau estimates of the 1994 population to calculate chronic disability prevalence rates adjusted for aging in the United States population aged >65. The 1982 age standardized rates were compared with 1994 NLTCS estimates. The prevalence of disability estimated for 1994 (21.3%) was 3.6% lower than the 1982 age standardized rate (24.9%)—a highly significant reduction (t = −8.5; P ≪ 0.0001). Of the 3.6 percentage point decline in prevalence, 1.7% occurred in the 5 years between 1989 and 1994—compared with the 1.9% decline in the 7 years between 1982 and 1989. Both declines are significant. Because of the shorter time period, the per year decline in disability prevalence from 1989 to 1994 was greater than that from 1982 to 1989. Given the higher acute and long-term care service needs of the disabled elderly population, Medicare, Medicaid, and private health expenditures may be dramatically lower than if declines had not occurred.
Keywords: longitudinal surveys, oldest–old, population aging
Changes in the prevalence of chronic disability in the United States elderly population are important for several reasons. First, many models of health changes in developed societies have suggested that industrialized, economically advanced countries would present social and public health problems that increase chronic disease and mortality risks (1, 2). When it was recognized in 1982 that United States chronic disease mortality rates above age 65 had been declining since at least 1968, with the consequence that the United States elderly population was growing faster than projected by the Social Security Administration (3, 4), the question was raised of whether chronic disease and disability rates had changed in a parallel fashion. If they had, one might expect the period of life afflicted with chronic morbidity and disability to remain relatively constant—or decline (5, 6). On the other hand, if chronic morbidity and disability incidence remained unchanged, with life expectancy increases above 65 largely due to improved medical treatments, then the period of life spent disabled might have increased (7). In this latter case, raising the normal retirement age for Social Security might not be a feasible solution to the problem. These questions played a major role in the debate about the changes in Social Security legislation finally passed in 1983. Because of a lack of clear evidence as to which scenario dominated health changes from 1968 to 1983, and which scenario would be likely to dominate in the future, a mixed strategy of a small increase in the normal retirement age (from 65 to 67 between the years 2003 and 2027) and increased payroll taxes was adopted. These policy options have to be revisited before large numbers of post-World War II baby boom cohorts pass the ages of 65 to 67 between the years 2011 and 2030.
Second, chronic disability, especially for elderly populations, is a sensitive measure of age-related changes in the health and biological fitness of individuals. It is a crucial question about the age rate of physiological changes, of whether recent increases in United States life expectancy at ages >65, and apparent changes in the manifest lifespan [i.e., the highest age to which individuals in a population are observed to live (8, 9)] are associated with not only reductions of chronic disease prevalence, but also increases in the average level of physical functioning at specific late ages. In this sense, disability is a marker of whether life expectancy increases are associated with changes in the age rate of loss of the average biological fitness of a population—a marker that could be interpreted as measuring changes in the biological rate of aging (10). This measure would be useful to help assess whether changes in the biological rate of aging, as inferred from models of the force of mortality [e.g., changes in the shape parameter of the Gompertz hazard function (11, 12); in some cases changes in the shape parameter is conditional on risk factor dynamics (13)], correlate with changes in the biological fitness of the United States elderly population as reflected in age-specific disability rates calculated from longitudinal survey data.
The arguments for using disability measures to make these assessments are substantive and methodological. Substantively, the dimensions of chronic disability are not only a product of chronic morbidity but are, increasingly, at advanced ages, primary risk factors for diseases such as stroke (14), coronary heart disease, peripheral vascular disease, diabetes, and cancer (15–18). Specifically, in middle age, disability is often viewed as a product of the age-related progression of the severity of specific chronic disease processes. At late ages (e.g., starting at 65) chronic disability will increasingly be a result of (i) the interaction of multiple disease processes (rather than the product of a single disease process) and/or (ii) the product of more general losses of physiological functions due to global processes of senescence (19). For example, declines in immune function with age increase the risk of pulmonary viral and bacterial infections leading to pneumonia. Decreased physical activity will lead to deconditioning of cardiopulmonary function, general vascular tone, and the fitness of voluntary muscles that may impair the efficiency of peripheral vascular function (10, 18, 20, 21). Thus, current reductions in the prevalence of chronic disability may affect the future prevalence of chronic disease and mortality risk in the United States elderly population.
Changes in chronic disability are very important for the United States health care system, because persons with chronic disabilities tend to have higher per capita Medicare, Medicaid, and other acute and chronic care health costs than nondisabled persons (22–24). Thus, reductions in chronic disability at late ages may have important direct and indirect effects on the future rate of growth of United States health care expenditures.
Methodologically, longitudinal measurements of chronic disability are important because they are easier to assess in nationally representative population surveys than many physiological risk factors or biomarkers of aging, which require blood drawing or other physically invasive (and expensive) tests and procedures (25, 26). The information content of longitudinal population monitoring can be greatly enhanced by linking an individual’s survey data to his/her continuous records of Medicare Part A and B service use. They also provide a temporal matrix for integrating data from specialized health studies—an integration that increases the value of that specialized health information by helping to assess, and possibly enhance, its population and temporal representativeness (27–29).
Though chronic disability time series are important to monitor age-specific changes in population health and biological fitness, measurement of chronic disability, especially in elderly populations, is complicated by the different types and degrees of disability manifest (13). There is no universal “gold standard” for measuring disability (30, 31). Measurement is less difficult for severe disability, where physical manifestations are readily observed and evaluated. Despite these methodological issues, measurements of disability are surprisingly robust, with national estimates of the prevalence of chronic, severe disability in the elderly shown to agree across several national surveys, even with differences in questionnaire wording, in a large federal interagency study (32). If assessed with the same instrument over time, and using similarly structured and coordinated samples, the likelihood of bias in estimates of prevalence change would be further reduced.
In this study, the prevalence of chronic disability and institutionalization in the elderly Unites States population was assessed for 1994 using the 1982 and 1994 National Long Term Care Surveys (NLTCS). Disability prevalence rates for 1982 were standardized to the July 1, 1994 Unites States population age distribution (33). The 1982 standardized rates were then compared with the 1994 observed rates to ascertain the size and direction of disability changes from 1982 to 1994 in the United States elderly population. This extends prior studies of the NLTCS, which documented significant declines in chronic disability from 1982 to 1989 (34, 35)
DATA
The data analyzed are individual reports of chronic (lasting, or expected to last, >90 days) impairments in activities of daily living (36) (ADL), instrumental activities of daily living (37) (IADL), and institutional residence from the 1982 and 1994 NLTCS. In each survey the same disability and medical condition questions were asked using identical field procedures and by the same survey organization (United States Census Bureau). This minimizes bias in disability trend estimates by holding constant instrument and field procedure, and related measurement artifact. The likelihood of bias is also reduced by the high (95%) response rates in all four NLTCS.
The samples for both NLTCS examined are large (i.e., 20,485 in 1982 and 19,171 in 1994) and designed to represent with precision the traits of the oldest–old population aged >85, a subgroup with high chronic disability prevalence rates, in each year. New samples of ≈5000 persons who attained age 65 between each pair of surveys were drawn from Medicare files in 1984, 1989, and 1994 to represent the United States population aged >65, in each year. In the 1994 NLTCS, screening is done of persons in the new sample component and of persons who were not disabled in prior samples, to identify chronic disability incident between 1989 and 1994. The samples, being drawn from Medicare enrollment files, are nationally representative of both community and institutional residents. Persons who receive a detailed interview in one survey year are automatically interviewed in all subsequent surveys until death. Because persons are followed through the Medicare record system, nearly 100% of cases can be longitudinally tracked so declines, as well as increases, in disability in previously disabled individuals can be identified, as well as exact dates of death.
In analyses of both the 1982 and 1994 NLTCS, United States Census Bureau cross-sectional sample weights were used. In 1989, the definition of institutional residence was expanded by the Census Bureau so that estimates of change in the institutional population may be conservative.
METHODS
Age (by 5-year categories from 65 to >95)-specific rates were calculated for persons with chronic disabilities, or who were institutionalized, in the 1982 and 1994 NLTCS. Disability was defined as the inability to perform ⩾1 IADL (e.g., cooking, doing laundry) due to health or aging, or the inability to perform at least one ADL (e.g., bathing, dressing) without using personal assistance or special equipment. Because institutional residents report an average of 4.8 ADLs impaired, they were used to define a separate high disability group. To be identified as chronically disabled when initially selected for a detailed interview a sample person had to have at least one ADL or IADL disability that had lasted, or was expected to last, >90 days. Disability was grouped into five categories, i.e., those with ⩾1 IADL impaired (but no ADL impaired), those with 1–2, 3–4, or 5–6 ADLs impaired, or persons residing in institutions reporting disability. All other persons were defined as not disabled.
Age-specific disability prevalences from the 1982 NLTCS were applied to Census Bureau age-specific population estimates for July 1, 1994 (33). Standard errors for the proportions were calculated from respondent counts. Changes in proportions were assessed using a test of differences in binomial probabilities.
RESULTS
In Table 1 we present the observed distribution of chronic disability in 1994 and the distribution of chronic disability that would have occurred in 1994 if the 1982 age-specific rates had not changed.
Table 1.
United States disability prevalence estimates for 1994 calculated from 1982 and 1994 NLTCS
| Functional status | 1994 prevalence from
|
|||
|---|---|---|---|---|
| 1982 rates, % | 1994 rates, % | Difference, % | t-value | |
| IADL impaired | 5.6 (±0.17)* | 4.3 (±0.14) | −1.3 (±0.22)† | −5.9 |
| 1–2 ADLs | 6.6 (±0.18) | 5.9 (±0.16) | −0.7 (±0.24) | −3.0 |
| 3–4 ADLs | 2.9 (±0.12) | 3.2 (±0.12) | 0.3 (±0.17) | 1.6 |
| 5–6 ADLs | 3.6 (±0.13) | 2.8 (±0.12) | −0.8 (±0.18) | −4.3 |
| Institutional resident | 6.3 (±0.18) | 5.2 (±0.16) | −1.1 (±0.24) | −4.5 |
| Total disabled | 24.9 (±0.31) | 21.3 (±0.29) | −3.6 (±0.42) | −8.5 |
Confidence bounds for proportion based on its SE.
Confidence bounds of differences in proportions based on its SE.
The proportion of the United States population aged >65 that would have been chronically disabled or institutionalized in 1994, had 1982 age-specific disability rates not changed, is 24.9%. The proportion observed to be chronically disabled in 1994 was 21.3%.
The United States chronically disabled elderly population in 1994 is 3.6% smaller (i.e., a decline from 24.9 to 21.3%), than if the 1982 chronic disability prevalence rates had not changed. This is a relative decline from 1982 of 14.5% in the 1994 disability prevalence rates. The decline of 3.6% is highly significant (t = −8.5; P ≪ 0.0001). The two standard error confidence bounds for the decline are 2.8 and 4.4%. The lower confidence bound (2.8%) is 50% greater than the 1.9% age standardized decline observed from 1982 to 1989. Thus, the decline in the prevalence of disability from 1982 to 1994 is significantly larger than the 1982 to 1989 decline.
In absolute terms, the change in prevalence suggests that there are 1.2 million fewer disabled persons in 1994 (i.e., 8.3 million vs. 7.1 million) than if the 1982 rates had not changed. Declines occurred at all but one level of disability. The disability level specific declines are each highly significant (P < 0.0001). The one increase in chronic disability prevalence, for persons with 3–4 ADLs disabled (0.3%), was not significant (t = 1.6; P > 0.10).
Changes for persons aged 65–74, 75–84, and >85 are presented in Table 2 for the nondisabled, those with only IADLs impaired, and those with at least one ADL impaired, or who are institutional residents.
Table 2.
Age-specific estimates of chronic disability prevalence in 1994 based on rates estimated from the 1982 and 1994 NLTCS
| Disability and age status | 1994 prevalence based on
|
|||
|---|---|---|---|---|
| 1982 rates, % | 1994 rates, % | Difference, % | t-value | |
| Nondisabled | ||||
| 65–74 | 85.9 (±0.33)* | 88.5 (±0.37) | 2.6 (±0.50)† | 5.3 |
| 75–84 | 68.1 (±0.56) | 73.1 (±0.47) | 5.0 (±0.74) | 6.8 |
| >85 | 34.8 (±0.94) | 40.2 (±0.88) | 5.4 (±1.29) | 4.2 |
| Only IADLs impaired | ||||
| 65–74 | 4.3 (±0.19) | 3.1 (±0.20) | −1.2 (±0.28) | −4.2 |
| 75–84 | 7.2 (±0.31) | 5.5 (±0.24) | −1.7 (±0.40) | −4.3 |
| >85 | 7.9 (±0.53) | 7.2 (±0.46) | −0.7 (±0.70) | −1.0 |
| ADL impaired or institutional | ||||
| 65–74 | 9.8 (±0.28) | 8.4 (±0.32) | −1.5 (±0.43) | −3.4 |
| 75–84 | 24.7 (±0.52) | 21.4 (±0.44) | −3.5 (±0.68) | −4.8 |
| >85 | 57.3 (±0.98) | 52.7 (±0.89) | −4.7 (±1.32) | −3.5 |
Confidence bounds for proportion based on its SE.
Confidence bounds of the difference in proportions based on its SE.
Differences between the proportions of the elderly population who were not chronically disabled in 1994 and who would be expected not to be disabled in 1994 based on 1982 rates, increased with age, i.e., 2.6, 5.0, and 5.4% for ages 65–74, 75–84, and >85, respectively. The declines in the proportion with ⩾1 IADL impaired were significant at ages 65–74 and 75–84. The decline (0.7%) at ages >85 was not significant (t = −1.0). The decline of the proportion of the population with ⩾1 ADL impaired, or in institutional residence, was significant at all ages (i.e., 1.5%, 3.3%, and 4.7% for ages 65–74, 75–84 and >85, respectively). Often, long-term care (LTC) insurance policies require a person to have ⩾3 ADLs impaired (not shown) before qualifying for benefits. The proportion with ⩾3 ADLs impaired declined significantly from 1982 to 1994, both overall (1.6%) and for specific age groups, i.e., declines of 1.0%, 2.5%, and 2.4% for ages 65–74, 75–74, and >85, respectively.
The 5000 person sample drawn in 1994 to represent persons passing age 65 between 1989 and 1994 is independent of the subsample of persons aged 65 to 69 in the 1982 NLTCS. Disabled survivors to 1994, who were aged >65 in 1982, had to be at least age 77 in 1994. Thus, declines in disability between the group aged 65 to 69 in 1982, and the same age group in 1994, are independent of the effects of previously responding to an interview (a measurement effect likely small due to the 5-year period between surveys). The proportion of nondisabled persons aged 65 to 69 was 88.3% in 1982 and 90.3% in 1994—a significant increase of 2.0% (t = 3.4). The group of persons aged 65 to 74 in 1982 is also independent of the group aged 65 to 74 in 1994. The increase in the proportion of nondisabled persons for that age group is larger (2.62%) and highly significant (t = 5.34). The decline between 1982 and 1994 (from 4.6 to 3.8%) in persons aged 65 to 69 with ⩾3 ADLs impaired was significant (t = −2.0). Significant declines were observed to the oldest ages examined, e.g., the decline between 1982 and 1994 in the proportion of persons aged >95 who were chronically disabled was 7.77% (SE = 2.42%; t = −3.2). Thus, declines in chronic disability were widespread, being found in most age and disability level specific subgroups.
DISCUSSION
The NLTCS data show declines in disability prevalence, observed from 1982 to 1989, in the United States elderly population, continued to 1994. If declines from 1989 to 1994 occurred at the same rate as between 1982 and 1989, the prevalence decline over the 5 years would have been 1.36%—compared with the 1.7% observed. Thus, declines in disability observed from 1982 to 1989 not only continued, but accelerated, from 0.27% per year from 1982 to 1989 to 0.34% per year from 1989 to 1994. Declines were manifest for all ages, even at ages of >95, and for the highest levels of disability. This is consistent with declines in United States mortality observed at ages of >80 (38), and with the proposition that higher United States expenditures on LTC better meet the needs of the very elderly than the lower LTC expenditures, and less complete LTC service availability, in Japan (39, 40).
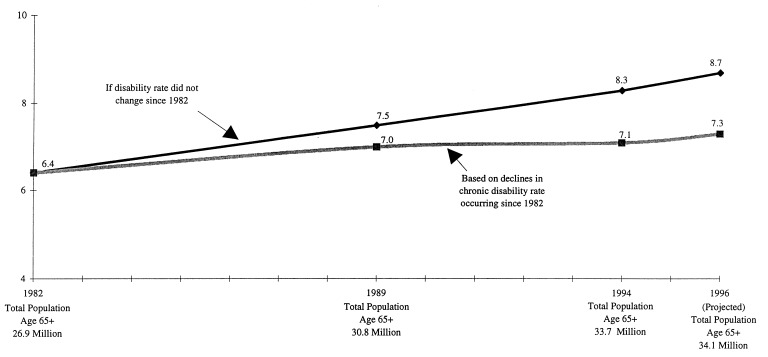
It is useful to examine what the prevalence rate changes from 1982 to 1994 imply for the number of disabled elderly persons at specific dates. This is shown in Fig. 1.
Figure 1.
Number of chronically disabled Americans aged 65 and over (in millions).
The 1982 United States population of 26.9 million persons aged 65+ had 6.4 million chronically disabled persons (35). By 1989 the number disabled grew 9.4% to 7.0 million persons—as opposed to the expected increase of 17.2% (14.5% due to the greater number of persons age 65+, and 2.7% due to the aging of the 65+ population). By 1994 the number chronically disabled had grown 10.9% to 7.1 million persons—rather than to 8.3 million persons, the 29.7% increase expected (25.3% due to the growth of the United States elderly population and 4.4% to the aging of that population) if the 1982 rates had not changed. If the 0.34% decline in disability per year (observed from 1989 to 1994) continued to 1996, the decline from 1982 to 1996 would be 4.3%. There would then be 7.3 million disabled persons in 1996 (an increase of 14.1%) compared with 8.7 million persons (an increase of 32.8%; 26.8% due to the growth of the 65+ population and 6.0% due to its aging) if the 1982 rates had not changed—a difference of 1.4 million persons.
Of the 1982 to 1994 decline in prevalence, two-thirds (2.4%) is due to changes in nonstandardized rates (i.e., the observed disability prevalence in 1982 was 23.7% compared with 21.3% in 1994). This 2.4% decline is over twice the decline (1.1%) in the nonstandardized rate changes (i.e., from 23.7 to 22.6%) from 1982 to 1989 (35). One-third of the change is due to the standardization used to adjust for aging of the United States elderly population. The sensitivity of the change estimate to the use of different standard populations was examined. Though the prevalence rates changed moderately when different standard populations (e.g., different intercensal estimates) were used, since the 1982 standardized and 1994 observed rates reflect the same 1994 population distribution, the change estimate is robust to the selection of a standard population.
In addition to a decline in the number of persons with chronic disability there was also a small decline (2.0%) in the average number of ADLs reported by persons who were ADL-disabled in both years. This reduction of 395,648 ADL impairments reported by the disabled population suggests that the population burden of disability was further reduced by an equivalent of 111,765 disabled persons—each with an average of 3.54 ADLs.
Two related issues of importance are (i) that the association of disability declines with changes in other measures of health and biological fitness at late ages and (ii) the identification of possible causes of the disability decline.
The declines in chronic disability are related to changes in other covariates in the NLTCS thought to be related to the likelihood of being chronically disabled at a given age. Education has been negatively related to the age-specific likelihood of being chronically disabled (24). The proportion of the population aged 85 to 89, with 0–7 years of education, has been estimated to decline dramatically, from >60% in 1980 to 10–15% by the year 2015 (41). It may be that this relation was due to only a select portion of the population receiving high levels of education in the past. As high levels of education become more prevalent, unless there is a causal relation, the ability of education to predict disability might be attenuated. Though such selection is a possible explanation, it would have to operate even though most schooling is done early in life (i.e., by age 30), whereas we are examining disability changes at ages >65. Because education is largely completed by age 30 this raises the question of what intermediate health care and biological mechanisms may be reducing the probability of disability for better educated persons. There are many candidate factors, i.e., better educated persons tend to more readily adopt healthy behaviors (e.g., maintaining physical activity at late ages, reducing risk factor exposures such as smoking, improving nutrition). Better educated persons are also more likely to have had better medical care both early in life and in middle age, up to age 65, when qualifying for Medicare—in part because of higher rates of health insurance coverage up to age 65 for better educated persons (42). Better educated persons are also better equipped to comply with complex and long-term medical treatments—especially for chronic conditions engendering comorbidities such as hypertension or diabetes. They are also more likely to exhibit fewer disabilities even with a chronic condition present (43, 44). Finally, there is evidence suggesting education is negatively associated with the incidence of Alzheimer’s disease—a prevalent and highly disabling condition (45).
An analysis of changes in disability levels by cohort (cohort effects were correlated with education) suggests that the age rate of disability increase (and, inferentially, of the biological rate of aging) is slower in younger, than older, cohorts. Since factors related to cohort membership are, by definition, persistent, this suggests that period differences in chronic disability are likely to continue past 1994, because those cohorts succeed one another at late ages where the general level of disability is high (70, 71).
Analysis of changes in 16 chronic diseases show that there were large declines in the prevalence of those conditions from 1982 to 1989 (35). To the extent that the progression of chronic disease increases chronic disability after a lag [say of 3–5 years for ages 75–79 (46, 47)], this is consistent with the continuation of the disability declines observed from 1989 to 1994. The decline in chronic diseases observed from 1982 to 1989 is, itself, consistent with trends in risk factors that showed, in the four National Health and Nutrition Examination Surveys done from 1960 to 1990, declines in hypertension, cholesterol levels, and smoking for persons aged 65 to 74 (48, 49). This is in contrast to Germany where a 19% decrease in cardiovascular disease mortality from 1984 to 1989 was argued by German researchers to be most likely due to improved medical therapy, because cardiovascular disease risk factors (50) and smoking (51) showed little or no improvement in studies done from 1970 to 1991.
Chronic disease and disability prevalence may have been declining in the United States for a much longer period of time than from 1982 to 1994. Fogel (52) found that heart disease prevalence, a disabling chronic disease, declined 6% per decade for 75 years from Civil War veterans evaluated at age >65 in 1910 to World War II veterans aged >65 in 1985 to 1988. Lanska and Mi (53) found stroke mortality (stroke being a disease engendering significant chronic disability) had declined since 1925.
Multiple changes in the questionnaires and samples used in the National Health Interview Surveys over time make long-term disability trends difficult to estimate (54) and comparisons with other surveys problematic (54–56). Waidmann et al. (54) attempted to resolve those difficulties by using a variety of survey and epidemiological data sets and making adjustments for known methodological problems. They found that not only did United States disability decline in the 1980s, but that apparent increases in disability in the 1970s were likely due to measurement difficulties with National Center for Health Statistics surveys done in that period (54–57). Administrative data also suggest that declines in institutional residence observed in the NLTCS are consistent with declines in Medicaid reimbursed nursing home use by the elderly (49), the tendency toward shorter nursing home stays with more medically intensive care, and the increased use in the United States of home health services for LTC (58).
Very long-term changes in chronic disease prevalence are less likely due to medical innovation than to changes in nutrition and public hygiene. A number of long-term trends in these factors possibly contributed to declines in chronic disease and disability. One factor is improvement in public hygiene and sanitation. A recently discovered pathogen, Helicobacter pylori, may explain long-term declines in gastric cancer (since the 1930s) and changes in peptic ulcer prevalence (59). Improvements in water quality may have reduced exposure to this pathogen. A second factor possibly contributing to long-term declines in stroke and gastric cancer is the reduced use of salt as a food preservative as refrigeration became more widely used (60). Indeed, salt consumption and H. pylori infection may interact to increase disease risk, as may salt and increased nitrate consumption. A third factor is better regulation of commercial food processing and livestock management. Commercial food processing, especially thermal processing of meats, may have reduced the prevalence of food-borne pathogens possibly contributing to atherosclerosis (61). Food processing expanded rapidly after 1950. Regulation of livestock feeding and handling may also have reduced food-borne viral pathogens. Fourth, food supplementation was initiated after the discovery in 1917 of the relation of cod liver oil consumption to rickets. Vitamin D supplementation, either by fish oil consumption or the UV radiation of milk, was widely spread by 1924 in the United States. British studies suggest that nutritional deficiencies in pregnancy may affect fetal development in ways to increase the risk of chronic disease in late adult life. Finally, a number of lifestyle factors could have contributed to changes. United States surgeon general reports in the early 1960s may have reduced smoking rates in successive birth cohorts (62).
The impact of medical therapy on disability prevalence is hard to assess because many treatment innovations for chronic diseases are recent [e.g., ACE-II inhibitors to control hypertension (63, 64)]. There are also relatively old medical interventions whose full range of effects on chronic disease and disability are just beginning to be understood. Exogenous estrogens were used in 1985 by 3 million United States women to treat postmenopausal symptoms. By 1995, nearly 10 million United States women were taking estrogens, which may have benefit for such highly prevalent, disabling conditions as osteoporosis and cardiovascular disease (65–67). A recent study suggested that exogenous estrogens might delay the onset of Alzheimer’s disease by 5–8 years—and possibly reduce the prevalence of that highly disabling condition in females by 50%. Aspirin may reduce the risk of colorectal cancer and recurrences of stroke and heart disease. Recent data suggest that nonsteroidal antiinflammatory drugs may also reduce the risk of Alzheimer’s disease—possibly by 25–40%. The effects of aspirin and other nonsteroidal antiinflammatory drugs, because of their widespread use, could affect the health of a large proportion of the United States elderly population. These older interventions may combine with numerous more recent medical advances to cause both chronic disease and disability to continue to decline for a long time—with the implication of exogenously altering basic biological parameters reflecting age changes.
Disability declines of the size in Fig. 1 may have important implications for national health care costs. For example, the 1994 United States institutional population was estimated to be 1.7 million persons. The 1982 rates, after age standardization, implied 2.1 million persons would be institutionalized in 1994. The difference of 400,000 implies, assuming an annual per capita nursing home cost in 1994 of $43,300 (49), savings of $17.3 billion in nursing home expenses in 1994. That does not include additional potential savings for acute care expenditures (22). Specifically, if age increase in the prevalence of chronic disability is a marker of the rate of loss of biological function, then decreases in the number of chronically disabled persons implies that persons in “predisabled” states are also losing biological function at a slower rate. Thus, declines in the number of disabled persons at late ages may imply better function and lower health care needs in a large proportion of the younger portion of the United States elderly population. If many acute care medical expenditures are due to chronic disability (22), then the decline in the prevalence of disability rates from 1989 to 1994, which caused the disabled elderly population to grow more slowly than the total elderly population, could portend slower rates of growth in national health expenditures in the future. A number of factors could affect this. The interpretation above assumed that the length of time a person remains disabled is relatively constant. In contrast, prevalence could decline if the same number of persons were disabled for shorter periods of time. This might seem reasonable in the context of declining life expectancy, i.e., that persons live a shorter period of time after a potentially disabling chronic disease became manifest. However, United States life expectancy has increased at later ages. If the prevalence decline were due to shorter disability episodes among the same number of persons this would require, in the context of increasing late age life expectancy, the proportion of the lifespan spent in nondisabled states to increase. That is, constraining the standard epidemiologic relation of prevalence to the incidence and duration of a chronic health event is an increasing late age life expectancy.
A change in the relation of disability duration and incidence could also have important effects on costs by changing the mix of medical services required. For example, institutional and informal care use would increase if the duration of disability increased. If duration of disability decreased, as health events increased in incidence, this might shift costs to hospital use and other types of acute health services.
Understanding the effects of chronic disability changes on the level and mix of health service expenditures is important in that currently there is great concern for the fiscal stability of Medicare. Beginning in 2001, the Medicare Trust Fund is projected to go into negative balance (69). Concerns about Medicare expenditures will increase until at least 2028 when the largest post-World War II baby boom cohorts pass age 65.
Unless gross domestic product grows faster than projected, the projected increases in the size of the elderly population relative to the labor force (and taxable payroll base) could require large changes in the Medicare program to maintain fiscal balance. However, projections of Medicare expenditures are based on the projected growth of the United States elderly population—with no assumptions made about how health changes before death in the calculations—even though life expectancy is projected to continue to increase at late ages. Thus, if declines in chronic disability continue, the magnitude of changes required in the Medicare (and Medicaid) benefits might be reduced because of two factors. One is the change in the ratio of the size of the United States population aged say, 18–64, to the population aged >65, who are chronically disabled. This differs from the standard dependency ratio of the 18–64 to >65 populations in that it would reflect changes in the aggregate health of the over 65 population—changes that could have large effects on Medicare and other health expenditures. For example, Medicare Part A and B expenditures are higher for chronically disabled persons. This is due to a variety of factors, e.g., persons with severe chronic disability are likely affected by medical conditions requiring considerable acute care. To maintain the health of a chronically disabled person, one thus has to provide large amounts of medical service to deal with morbidity engendered by the physiological effects of disability.
To keep a constant ratio between the United States population aged 18–64 as projected for 2028, to the number of disabled persons over 65, the disability prevalence rate above age 65 would have to be reduced 1.5% each year for 34 years, i.e., an overall reduction of 1 − (0.98530) = 0.402, or 40.2% in 34 years. The rate of decline from 1982 to 1994 has been 1.2% per year, so a 1.5% rate of decline in the disability prevalence rate is conceivable. However, it is important to recognize that after the peak in 2028, the size of the age >65 population relative to the population aged 18–64 declines. Hence, to maintain a constant ratio in 2050, the per annum rate of decline in the prevalence of disability above age 65 required is lower—roughly 1.2%, the same rate of decline observed from 1982 to 1994. Even if such rates of decline are not maintained over these longer periods, any sizeable decline will reduce the magnitude of Medicare and Medicaid program changes needed and possibly smooth out the demands induced at the times of the peak Medicare burden.
A second factor determining how rapidly Medicare’s burden will grow is that the per capita annual rate of Medicare’s expenditures declines with increasing age at death (68). This is because a large proportion of Medicare costs, at any age, are made in the 2 years immediately before death. The per capita expenditures for deaths at age 70 are $22,590, whereas for deaths at age >101 they decline to $8,300 (1990 dollars; 1989–1990 deaths). This age trajectory of expenditures, combined with the apparent slowing of biological age changes over time, may have important consequences for the future burden of the Medicare program. These were not factored into projections of Mediacre Trust Fund obligations (3, 22, 55, 69).
In summary, declines in the prevalence of chronic disability, observed up to age 95, suggest that there have been statistically significant and biologically important changes in the age rate of loss of biological fitness in the United States population associated with increases in life expectancy above age 65. It will be important to monitor these changes in chronic disability to determine how the age rates of loss of biological fitness will behave in the future. These changes may have important implications in forecasting the future trajectory of changes in health expenditures in the United States elderly population.
Acknowledgments
This research was supported by the National Institute on Aging.
ABBREVIATIONS
- NLTCS
National Long Term Care Surveys
- ADL
activities of daily living
- IADL
instrumental ADL
- LTC
long-term care
References
- 1.Dubos R. Man Adapting. New Haven, CT: Yale Univ. Press; 1965. [Google Scholar]
- 2.Omran A. Milbank Mem Q. 1971;49:509–538. [PubMed] [Google Scholar]
- 3.Singer B H, Manton K G. Focus. 1993;15:1–10. [Google Scholar]
- 4.Myers G C. In: Aging: A Challenge to Science and Social Policy. Gilmore A J, editor. Vol. 2. Oxford: Oxford Univ.; 1981. pp. 248–260. [Google Scholar]
- 5.Manton K G. Milbank Mem Fund Q. 1982;60:183–244. [PubMed] [Google Scholar]
- 6.Manton K G. In: The Annals of the American Academy of Political and Social Science. Riley M, Riley J, editors. Vol. 503. Newbury Park, CA: Sage; 1989. pp. 72–87. [Google Scholar]
- 7.Feldman J. Milbank Mem Fund Q. 1983;61:430–444. [PubMed] [Google Scholar]
- 8.Manton K G, Stallard E. J Gerontol Biol Sci. 1996;51:B362–B375. doi: 10.1093/gerona/51a.5.b362. [DOI] [PubMed] [Google Scholar]
- 9.Social Security Administration (1992) Actuarial Study 107, SSA Publ. No. 11–11536 (Social Security Administration, Baltimore).
- 10.Kasch F, Boyer J, Van Camp S, Verity L, Wallace J. Age Aging. 1993;22:5–10. doi: 10.1093/ageing/22.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Finch C. Longevity, Senescence, and the Genome. Chicago: Univ. of Chicago Press; 1990. [Google Scholar]
- 12.Finch C, Pike M. J Gerontol. 1996;51:B183–B194. doi: 10.1093/gerona/51a.3.b183. [DOI] [PubMed] [Google Scholar]
- 13.Manton K G, Stallard E, Woodbury M A, Dowd J E. J Gerontol. 1994;49:B169–B190. doi: 10.1093/geronj/49.4.b169. [DOI] [PubMed] [Google Scholar]
- 14.Colantonio A, Kasl S V, Ostfeld A M. Stroke. 1992;23:1355–1357. doi: 10.1161/01.str.23.9.1355. [DOI] [PubMed] [Google Scholar]
- 15.Blair S N, Kohl H W, Paffenbarger R S, Clark D G, Cooper K H, Gibbons L W. J Am Med Assoc. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 16.Lindsted K D, Tonstad S, Kuzma J W. J Clin Epidemiol. 1991;44:355–364. doi: 10.1016/0895-4356(91)90074-j. [DOI] [PubMed] [Google Scholar]
- 17.Paffenbarger R S, Hyde R T, Wing A L, Hsieh C C. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 18.Shinton R, Sagar G. Br Med J. 1993;307:231–234. doi: 10.1136/bmj.307.6898.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strehler B. Time, Cells and Aging. New York: Academic; 1977. [Google Scholar]
- 20.Drexler H, Reide S, Munzel T, Konig H, Funke E, Just H. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 21.Lakka T, Venalainen J, Rauramaa R, Salonen R, Tuomilehto J, Salonen J. N Engl J Med. 1994;330:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 22.Manton K G, Singer B H, Suzman R M, editors. Forecasting the Health of Elderly Populations. New York: Springer; 1993. [Google Scholar]
- 23.Manton K G. In: Health Care Management: State of the Art Reviews Series. Holt H, Leibovici M M, editors. Philadelphia: Hanley & Befus; 1996. in press. [Google Scholar]
- 24.Manton K G, Stallard E. J Aging Soc Policy. 1996;7:25–52. doi: 10.1300/J031v07n03_03. [DOI] [PubMed] [Google Scholar]
- 25.Kervinen K, Savolainen M, Salokannel J, Hynninen A, Heillinen J, Ehnholm C, Koistinen M, Kesaniemi Y. Atherosclerosis. 1994;105:89–95. doi: 10.1016/0021-9150(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 26.Strittmatter W, Saudners A, Schmechel D, Pericak-Vance M, Enghild J, Salvesen G, Roses A. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiatarone M, Marks E, Ryan N, Merdith C, Lipsitz L, Evans W. J Am Med Assoc. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 28.Fiatarone M, O’Neill E, Doyle N, Clements K, Roberts S, Kehayias J, Lipsitz L, Evans W. J Am Geriatr Soc. 1993;41:333–337. doi: 10.1111/j.1532-5415.1993.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 29.Fiatarone M, O’Neill E, Ryan N, Clements K, Solares G, Nelson M, Roberts S, Kehayias J, Lipsitz L, Evans W. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 30.Dorevitch M I, Cossar R M, Bailey F J, Bisset T, Lewis S J, Wise L A, MacLennan W J. J Clin Epidemiol. 1992;45:791–798. doi: 10.1016/0895-4356(92)90057-t. [DOI] [PubMed] [Google Scholar]
- 31.Reuben D, Siu A, Kimpau S. J Gerontol Med Sci. 1992;47:M106–M110. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 32.Wiener J M, Hanley R J, Clark R, Van Nostrand J F. J Gerontol. 1990;45:S229–S227. doi: 10.1093/geronj/45.6.s229. [DOI] [PubMed] [Google Scholar]
- 33.Day, J. C. (1993) Population Projections of the United States, by Age, Sex, Race, and Hispanic Origin: 1993 to 2050, Current Population Reports, Series P25–1104. (GPO, Washington, DC).
- 34.Manton K G, Corder L S, Stallard E. J Gerontol. 1993;47:S153–S166. doi: 10.1093/geronj/48.4.s153. [DOI] [PubMed] [Google Scholar]
- 35.Manton K G, Stallard E, Corder L S. J Gerontol. 1995;50:S194–S204. doi: 10.1093/geronb/50b.4.s194. [DOI] [PubMed] [Google Scholar]
- 36.Katz S, Akpom C A. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 37.Lawton M P, Brody E M. Gerontology. 1969;9:179–186. [PubMed] [Google Scholar]
- 38.Manton K G, Vaupel J W. N Engl J Med. 1995;333:1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto Y. Br Med J. 1992;305:403–405. [Google Scholar]
- 40.Ikegami N, Campbell J C. N Engl J Med. 1995;333:1295–1299. doi: 10.1056/NEJM199511093331922. [DOI] [PubMed] [Google Scholar]
- 41.Preston S. The Oldest Old. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 42.Frank P, Clancy C, Gold M. J Am Med Assoc. 1993;270:737–741. [PubMed] [Google Scholar]
- 43.Callahan L, Bloch D, Pincus T. J Clin Epidemiol. 1992;45:127–138. doi: 10.1016/0895-4356(92)90005-8. [DOI] [PubMed] [Google Scholar]
- 44.Hannan M, Anderson J, Pincus T, Felson D. J Clin Epidemiol. 1992;45:139–147. doi: 10.1016/0895-4356(92)90006-9. [DOI] [PubMed] [Google Scholar]
- 45.Stern Y, Gurland B, Tatemichi T, Tang M, Wilder D, Mayeux R. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 46.Law M, Wald N, Thompson S. Br Med J. 1994;308:367–373. [Google Scholar]
- 47.Law M, Thompson S, Wald N. Br Med J. 1994;308:373–379. doi: 10.1136/bmj.308.6925.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson C, Rifkind B, Sempos C, Carroll M, Bachorik P, Briefel R, Gordon D, Burt V, Brown C, Lippel K, Cleeman J. J Am Med Assoc. 1993;269:3002–3008. [PubMed] [Google Scholar]
- 49.National Center for Health Statistics. Health United States: 1994. Washington, DC: Public Health Service; 1995. [Google Scholar]
- 50.Hoffmeister H, Mensink G, Stolzenberg H. Prev Med. 1994;23:197–205. doi: 10.1006/pmed.1994.1027. [DOI] [PubMed] [Google Scholar]
- 51.Brenner H. J Epidemiol Community Health. 1993;47:54–58. doi: 10.1136/jech.47.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fogel R. Am Econ Rev. 1994;84:369–395. [Google Scholar]
- 53.Lanska D, Mi X. Stroke. 1993;24:1382–1388. doi: 10.1161/01.str.24.9.1382. [DOI] [PubMed] [Google Scholar]
- 54.Waidmann T, Bond J, Schoenbaum M. Milbank Mem Fund Q. 1995;73:253–287. [PubMed] [Google Scholar]
- 55.National Center for Health Statistics (1985) Vital and Health Statistics, Series 1, No. 18, DHHS Publ. No. (PHS) 85–1320 (Public Health Service, Washington, DC).
- 56.Wilson R, Drury J. Annu Rev Publ Health. 1984;5:83–106. doi: 10.1146/annurev.pu.05.050184.000503. [DOI] [PubMed] [Google Scholar]
- 57.Manton K G. In: Proceedings: Future Income and Health Care Needs and Resources for the Aged. Ross S G, Walker D M, editors. Baltimore: GPO; 1994. pp. 119–177. [Google Scholar]
- 58.Welch H G, Wennberg D E, Welch W P. N Engl J Med. 1996;335:324–329. doi: 10.1056/NEJM199608013350506. [DOI] [PubMed] [Google Scholar]
- 59.Sonnenberg A. Ailment Pharmacol Ther. 1995;9:3–12. [PubMed] [Google Scholar]
- 60.Joossens J V, Hill M J, Elliott P, Stamler R, Stamler J, Lesaffre E, Dyer A, Nichols R, Kesteloot H. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]
- 61.Mozar H N, Bal D G, Farag S A. Atherosclerosis. 1990;82:157–164. doi: 10.1016/0021-9150(90)90154-b. [DOI] [PubMed] [Google Scholar]
- 62.Harris J E. J Natl Cancer Inst. 1983;71:473–479. [PubMed] [Google Scholar]
- 63.Materson B, Preston R. Arch Intern Med. 1994;154:513–523. [PubMed] [Google Scholar]
- 64.Paul S, Kuntz K, Eagle K, Weinstein M. Arch Intern Med. 1994;154:1143–1149. [PubMed] [Google Scholar]
- 65.Nabulsi A, Folsom A, White A, Patsch W, Heiss G, Wu K, Szklo M. N Engl J Med. 1993;328:1069–1075. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
- 66.Bellantoni M, Harman S, Cullins V, Engelhardt S, Blackman M. J Gerontol Med Sci. 1991;46:M216–M222. doi: 10.1093/geronj/46.6.m216. [DOI] [PubMed] [Google Scholar]
- 67.Eaker E, Pinsky J, Castelli W. Am J Epidemiol. 1992;135:854–864. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 68.Lubitz J, Beebe J, Baker C. N Engl J Med. 1995;332:999–1003. doi: 10.1056/NEJM199504133321506. [DOI] [PubMed] [Google Scholar]
- 69.Board of Trustees, Federal Hospital Insurance Trust Fund. 1996 Annual Report. Washington, DC: Library of Congress; 1996. [Google Scholar]
- 70.Manton, K. G., Stallard, E. & Corder, L. (1997) Demography, in press. [PubMed]
- 71.Manton, K. G., Stallard, E. & Corder, L. (1997) J. Aging Health, in press. [DOI] [PubMed]