Abstract
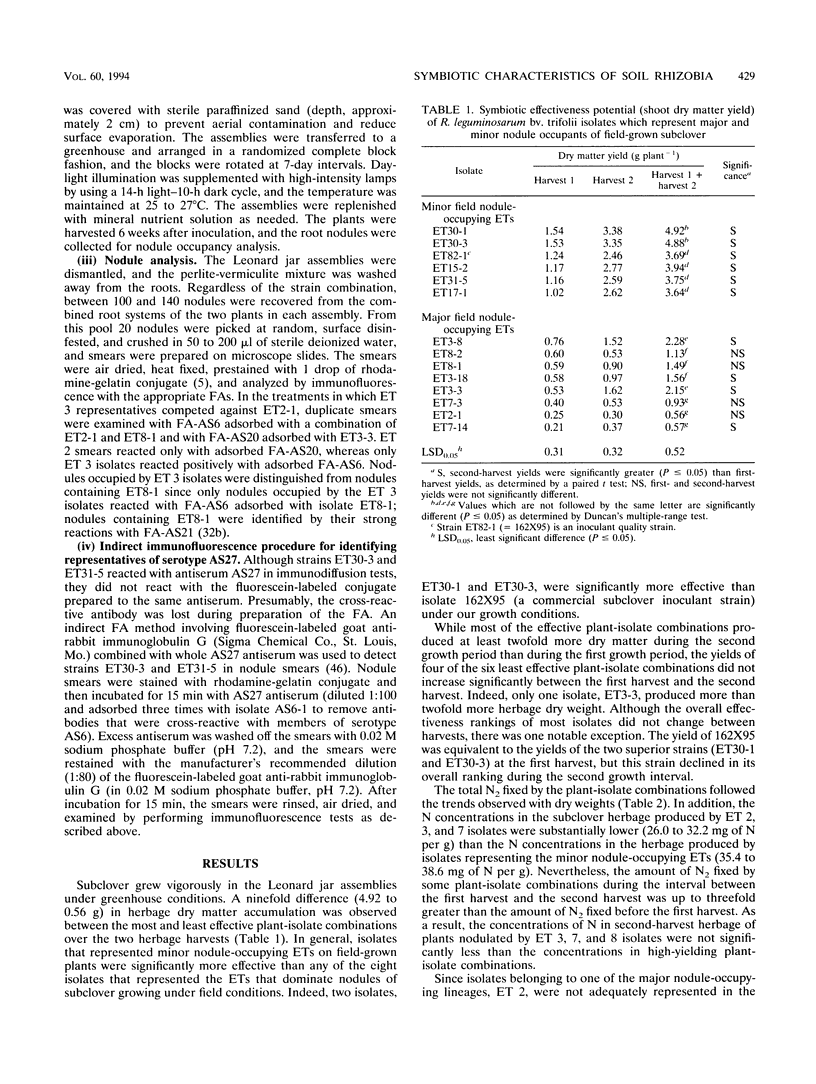
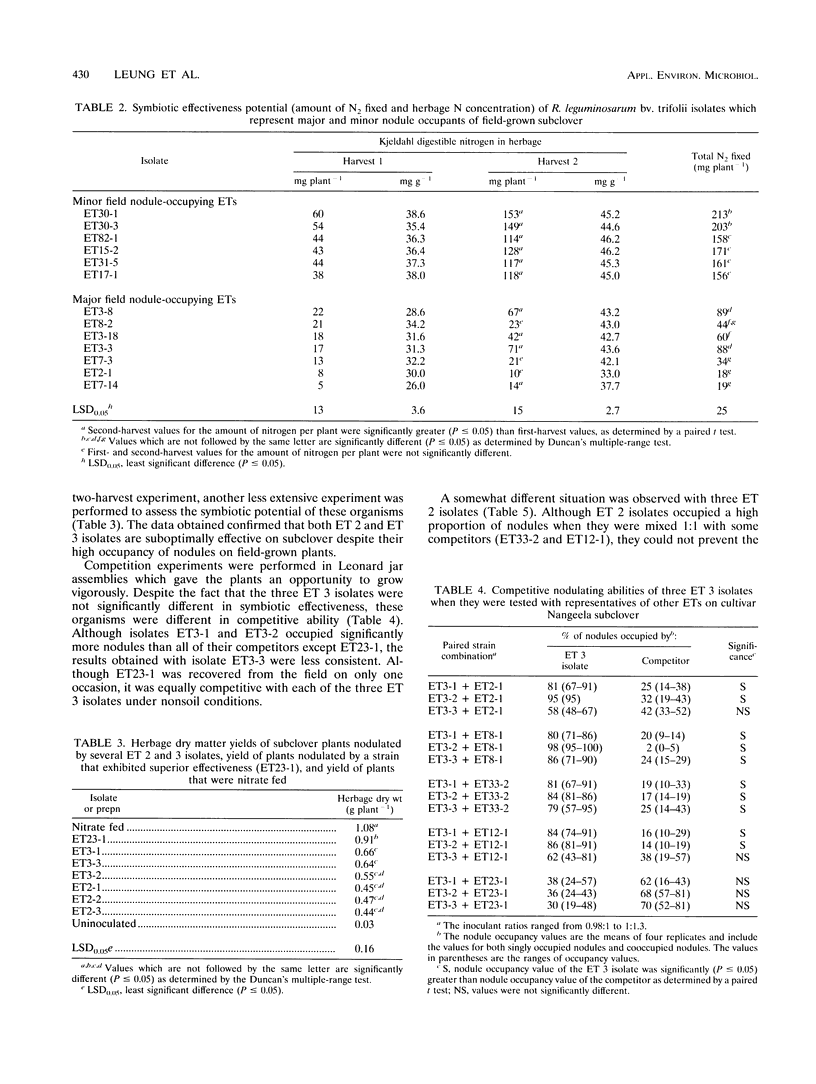
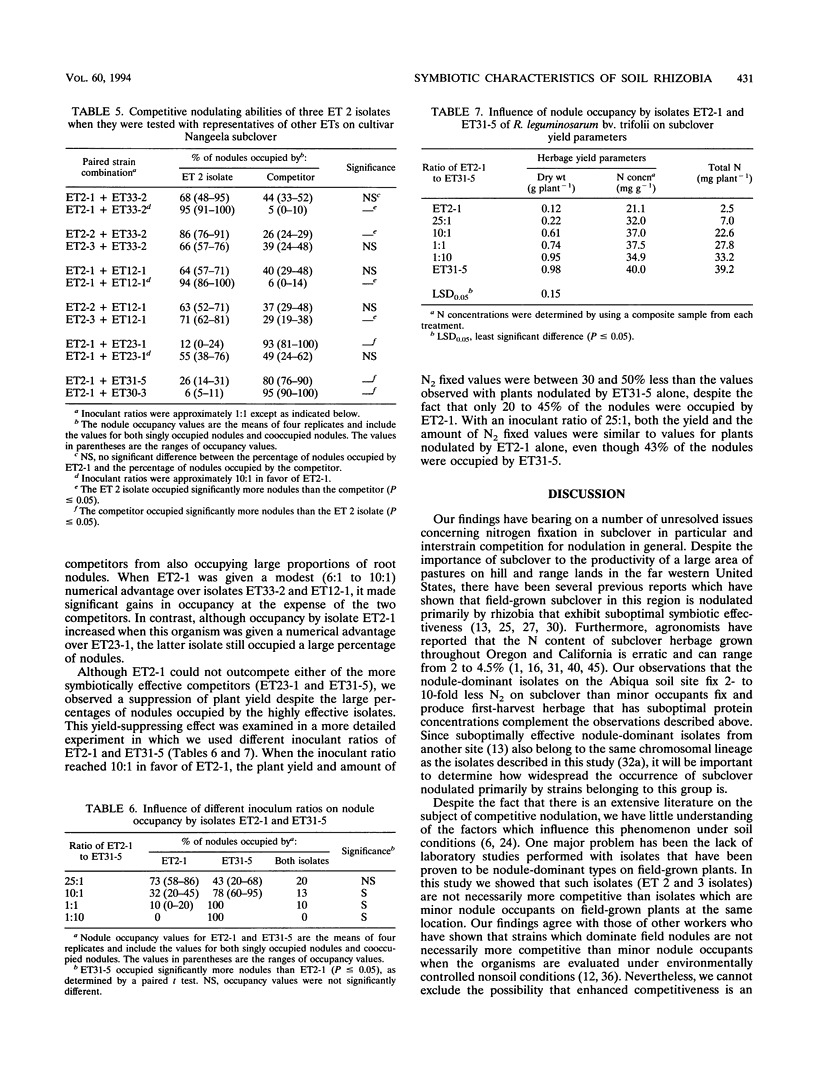
The symbiotic effectiveness and nodulation competitiveness of Rhizobium leguminosarum bv. trifolii soil isolates were evaluated under nonsoil greenhouse conditions. The isolates which we used represented both major and minor nodule-occupying chromosomal types (electrophoretic types [ETs]) recovered from field-grown subclover (Trifolium subterraneum L.). Isolates representing four ETs (ETs 2, 3, 7, and 8) that were highly successful field nodule occupants fixed between 2- and 10-fold less nitrogen and produced lower herbage dry weights and first-harvest herbage protein concentrations than isolates that were minor nodule occupants of field-grown plants. Despite their equivalent levels of abundance in nodules on field-grown subclover plants, ET 2 and 3 isolates exhibited different competitive nodulation potentials under nonsoil greenhouse conditions. ET 3 isolates generally occupied more subclover nodules than isolates belonging to other ETs when the isolates were mixed in 1:1 inoculant ratios and inoculated onto seedlings. In contrast, ET 2 isolates were less successful at nodulating under these conditions. In many cases, ET 2 isolates required a numerical advantage of at least 6:1 to 11:1 to occupy significantly more nodules than their competitors. We identified highly effective isolates that were as competitive as the ET 3 isolates despite representing serotypes that were rarely recovered from nodules of field-grown plants. When one of the suboptimally effective isolates (ET2-1) competed with an effective and competitive isolate (ET31-5) at several different inoculant ratios, the percentages of nodules occupied by the former increased as its numerical advantage increased. Although subclover yields declined as nodule occupancy by ET2-1 increased, surprisingly, this occurred at inoculant ratios at which large percentages of nodules were still occupied by ET31-5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarger N., Lobreau J. P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982 Sep;44(3):583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames-Gottfred N. P., Christie B. R. Competition among Strains of Rhizobium leguminosarum biovar trifolii and Use of a Diallel Analysis in Assessing Competition. Appl Environ Microbiol. 1989 Jun;55(6):1599–1604. doi: 10.1128/aem.55.6.1599-1604.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie G. A., Clayton M. K., Handelsman J. Quantitative comparison of the laboratory and field competitiveness of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1989 Nov;55(11):2755–2761. doi: 10.1128/aem.55.11.2755-2761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Brom S., García de los Santos A., Stepkowsky T., Flores M., Dávila G., Romero D., Palacios R. Different plasmids of Rhizobium leguminosarum bv. phaseoli are required for optimal symbiotic performance. J Bacteriol. 1992 Aug;174(16):5183–5189. doi: 10.1128/jb.174.16.5183-5189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom S., García de los Santos A., de Lourdes Girard M., Dávila G., Palacios R., Romero D. High-frequency rearrangements in Rhizobium leguminosarum bv. phaseoli plasmids. J Bacteriol. 1991 Feb;173(3):1344–1346. doi: 10.1128/jb.173.3.1344-1346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield E. S., Lewis D. M., Barran L. R. Cryptic plasmid and rifampin resistance in Rhizobium meliloti influencing nodulation competitiveness. J Bacteriol. 1985 Oct;164(1):410–413. doi: 10.1128/jb.164.1.410-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Interstrain Competition between Representatives of Indigenous Serotypes of Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1020–1025. doi: 10.1128/aem.52.5.1020-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling D. N., Broughton W. J. Competition for nodulation of legumes. Annu Rev Microbiol. 1986;40:131–157. doi: 10.1146/annurev.mi.40.100186.001023. [DOI] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M. L., Robert F. M. Competition among Rhizobium leguminosarum bv. phaseoli strains for nodulation of common bean. Can J Microbiol. 1992 Feb;38(2):157–160. doi: 10.1139/m92-026. [DOI] [PubMed] [Google Scholar]
- Leung K., Strain S. R., de Bruijn F. J., Bottomley P. J. Genotypic and Phenotypic Comparisons of Chromosomal Types within an Indigenous Soil Population of Rhizobium leguminosarum bv. trifolii. Appl Environ Microbiol. 1994 Feb;60(2):416–426. doi: 10.1128/aem.60.2.416-426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Yap K., Dashti N., Bottomley P. J. Serological and Ecological Characteristics of a Nodule-Dominant Serotype from an Indigenous Soil Population of Rhizobium leguminosarum bv. trifolii. Appl Environ Microbiol. 1994 Feb;60(2):408–415. doi: 10.1128/aem.60.2.408-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner H. H., Strijdom B. W., Law I. J. Unaltered Nodulation Competitiveness of a Strain of Bradyrhizobium sp. (Lotus) after a Decade in Soil. Appl Environ Microbiol. 1989 Nov;55(11):3000–3008. doi: 10.1128/aem.55.11.3000-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Romero Esperanza, Rosenblueth Monica. Increased Bean (Phaseolus vulgaris L.) Nodulation Competitiveness of Genetically Modified Rhizobium Strains. Appl Environ Microbiol. 1990 Aug;56(8):2384–2388. doi: 10.1128/aem.56.8.2384-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J., Higgins P., O'gara F. Studies on the Inoculation and Competitiveness of a Rhizobium leguminosarum Strain in Soils Containing Indigenous Rhizobia. Appl Environ Microbiol. 1985 Apr;49(4):899–903. doi: 10.1128/aem.49.4.899-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D., Brom S., Martínez-Salazar J., Girard M. L., Palacios R., Dávila G. Amplification and deletion of a nod-nif region in the symbiotic plasmid of Rhizobium phaseoli. J Bacteriol. 1991 Apr;173(8):2435–2441. doi: 10.1128/jb.173.8.2435-2441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Barta T. M. Trifolitoxin Production and Nodulation Are Necessary for the Expression of Superior Nodulation Competitiveness by Rhizobium leguminosarum bv. trifolii Strain T24 on Clover. Plant Physiol. 1987 Oct;85(2):335–342. doi: 10.1104/pp.85.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Sadowsky M. J. Genetics of competition for nodulation of legumes. Annu Rev Microbiol. 1992;46:399–428. doi: 10.1146/annurev.mi.46.100192.002151. [DOI] [PubMed] [Google Scholar]



