Abstract
Neuronal development requires several discrete morphological steps that are believed to involve the small GTPase Rac. For example, neural activity, through NMDA receptors and/or AMPA receptors, activates Rac leading to elaboration of dendritic arbors. In the current study, we have conducted studies which indicate that Rac might be an important molecule involved in morphological plasticity in the adult mouse. We demonstrate that Rac is expressed at synapses in the adult mouse hippocampus. We also demonstrate that treatment of hippocampal slices with NMDA induces membrane translocation and activation of Rac in area CA1. Interestingly, we also find that there is an increase in Rac that is associated with NMDA receptor complexes following NMDA receptor activation. Taken together, our data are consistent with the idea that Rac could be participating in NMDA receptor-dependent changes in morphology that occur during synaptic plasticity and memory formation in the adult mouse hippocampus.
Keywords: Small GTP-binding proteins, Synaptic plasticity, Learning, Rho subfamily, CA1
The role of small GTP-binding proteins as signaling molecules has been studied extensively in many cell types, but their function is somewhat less understood in the field of neuroscience. The small G-proteins cycle between inactive (GDP-bound) and active (GTP-bound) conformations through the catalytic action of guanine exchange factors (GEF) and GTPase-activating proteins (GAP) [1]. In a second cycle, the small G-proteins cycle between cytosolic and membrane-associated forms [2] through the action of guanine dissociation inhibitor (GDI) proteins. How these two cycles are coupled is critical for the proper interaction of G-proteins with their targets [3]. One member of the Rho GTPase subfamily, Rac, has been implicated in regulation of numerous cellular processes [4,5]. Rac is probably best known for its role in regulating actin polymerization and the assembly of associated integrin complexes [6]. Rac also has a role in gene transcription [7] and G1 cell cycle progression [8].
Neuronal development involves several discrete morphological steps requiring migration of newborn neurons to specific locations, extension of axons and dendrites into proper target regions, and formation of synapses with appropriate partners. Small G-proteins including Rac are believed to be critical regulators of these processes [3,9]. Moreover, it has been proposed that Rho GTPase-mediated dendritic arbor growth induced by neural activity is via NMDA receptors and/or AMPA receptors, resulting in the activation of Rac and inactivation of RhoA [10]. There is very strong evidence in cultured neurons from rats that glutamatergic synaptic activity activates Rac [11–14] and the subsequent growth of dendritic arbors [10,15].
In this manuscript, we investigated regulation of Rac in the adult mouse hippocampus as a first step to determine whether Rac might be involved in the morphological changes associated with synaptic plasticity and memory formation. Our findings indicate that activation of NMDA receptors results in membrane translocation and activation of Rac, consistent with the idea that Rac plays a role in adult synaptic plasticity and memory.
Materials and methods
Brain homogenates
Animals were handled and sacrificed in compliance with institutional and national regulations and policies. The protocol was approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Male C57BL6 mice (3–5 weeks old) were sacrificed by cervical dislocation under gentle anesthesia. The brain was removed and cortex, cerebellum, hippocampus, and brain stem were dissected and homogenized in buffer (HBC; 10 mM Hepes, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 μM microcystin-LR, and 200 nM calyculin A). The homogenates were centrifuged at 1500g for 10 min at 4 °C to eliminate debris. Total protein concentrations were determined [16].
Hippocampal slice preparation and drug treatments
Hippocampi from mice were removed and 400 μm slices were prepared using a vibratome. Slices were perfused for 1 h with a standard saline solution (5 mM glucose, 110 mM sucrose, 60 mM NaCl, 28 mM NaHCO3, 3 mM KCl, 1.25 mM NaH2PO4, 7 mM MgCl2, 0.5 mM CaCl2, and 0.6 mM ascorbate) and equilibrated with 95% O2/5% CO2. For pharmacological experiments, slices were transferred to individual chambers and maintained in artificial cerebrospinal fluid (ACSF, 25 mM glucose, 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, and 2 mM CaCl2) for 1 h at 32 °C and exposed to either the corresponding drug for the indicated times (NMDA 100 μM for 3 min, DHPG 50 μM for 5 min, KCl 90 mM for 5 min, APV 50 μM for 10 min, MK801 50 μM for 10 min or TTX 1 μM for 10 min) or vehicle control (ACSF). Area CA1 was isolated immediately after treatments and homogenized in HBC. Homogenates were then centrifuged at 100,000g for 45 min using an air-driven ultracentrifuge (Beckman Coulter, Palo Alto, CA) and separated into membrane and cytoplasmic fractions. Total protein concentrations were determined [16]. Presence of Na+/K+ ATPase was tested to determine the purity of the membrane fractions.
Synaptoneurosome and postsynaptic density preparation
Synaptoneurosome and postsynaptic densities (PSDs) were isolated as described previously [17,18] with some modifications. Briefly, hippocampi from 5-week-old C57BL/6 male mice were homogenized in synaptoneurosome buffer (10 mM Hepes, 1 mM EDTA, 2 mM EGTA, 0.5 mM DTT, 10 μg/ml leupeptin, and 50 μg/ml soybean trypsin inhibitor) at 4 °C, followed by sonication. The samples then were filtered (100 μm, 30 μm, and finally 5 μm filter, Millipore Co., Bedford, MA). The filtrates were pooled together and centrifuged for 10 min at 10,000g at 4 °C. The resulting pellet containing synaptoneurosomes was thoroughly resuspended in solution B (1:6 w/v, 0.32 M sucrose, and 1 mM NaHCO3). A portion of these synaptoneurosomes was saved and analyzed via Western blots. The remaining sample was resuspended in an equal volume of 1% Triton X-100 in 32 mM sucrose–12 mM Tris–HCl (pH 8.1) and centrifuged for 20 min at 33,000g at 4 °C. The pellet was suspended in 0.5 ml of solution B, briefly sonicated, and layered onto a sucrose gradient containing 1.3 ml of 2 M sucrose, 1 ml of 1.5 M sucrose–1 mM NaHCO3, and 1 ml of 1 M sucrose–1 mM NaHCO3. Sample was then centrifuged for 2–4 h at 200,000g at 4 °C. The PSD containing band between the 2 M sucrose and 1.5 M sucrose–1 mM NaHCO3 layers was extracted, transferred to a tube, and diluted with 600 μl of solution B and 600 μl of 1% Triton X-100–150 mM KCl. The sample then was centrifuged at 200,000g for 12 min at 4 °C. The resulting pellet was suspended in solution B and sonicated. The PSD protein yield was calculated [16].
Rac activation assay
Twenty micrograms of GST-tagged PAK–PBD protein beads (Cytoskeleton, Denver, CO) was added to 150–200 μg protein of hippocampal homogenates containing protease inhibitor cocktail (Sigma, St. Louis, MO). Homogenates were incubated at 4 °C on a rocker for 1 h. PAK–PBD–GST beads then were pelleted by centrifugation at 5000g for 5 min at 4 °C. The supernatant was removed and the pelleted beads were washed with a phosphate-buffered saline solution. PAK–PBD–GST beads were pelleted again by centrifugation at 5000g for 5 min at 4 °C. The supernatant was removed again followed by two more washes and centrifugations. The final pellet was resuspended in 20μl Laemmli buffer, and analyzed by SDS–PAGE and Western blot analysis. Several controls were loaded in each gel in parallel to the affinity-purified samples to validate the assay.
Immunoprecipitation
Fifty microliters of 4× RIPA buffer (0.6 M NaCl, 4% Triton X-100, 4% sodium deoxycholate, 0.4% SDS, 100 mM Tris–HCl, pH 7.5, 20 μg/ml leupeptin, 20 μg/ml aprotinin, and 4 mM PMSF) was added to 200 μg protein for each homogenate to solubilize the sample for 10 min at 0 °C. Samples then were diluted three times with a Tris–HCl, buffer solution (TBS, 50 mM Tris–HCl, pH 7.5, 150 mM NaCl) and centrifuged at 12,000g for 10 min at 4 °C and the supernatants were collected. A molar excess of the corresponding primary antibody (4–8 μg of purified antibody against Rac and/or NMDA-receptor subunit NR1) was added to the protein solution containing the antigen of interest. Samples were incubated overnight at 4 °C in a rotator. Immobilized protein A (Pierce Biotechnology, Rockford, IL) was added to the antigen–antibody complex (~50 μl/5 μg of antibody) followed by incubation with gentle mixing in a rotator for 1–2 h at room temperature. Samples were centrifuged at 12,000g for 10 min at 4 °C. The immobilized protein A-bound complexes were washed twice with 1× RIPA buffer followed by centrifugation at 12,000g for 10 min at 4 °C. Supernatants were discarded and complexes were washed three times with TBS followed by centrifugation. Bound antigen–antibody complex from the immobilized protein A was resuspended in 30–50μl of SDS–PAGE sample buffer and then incubated for 5 min at 95 °C. Samples were characterized by SDS–PAGE and Western blot analysis.
Hippocampal CA1/CA3 dissociated cultures
Hippocampal cell cultures were prepared as previously described [19]. Cultures were maintained at 37 °C in 5% CO2 humidified incubator.
Immunocytochemistry
Four-week-old neuronal cultures from mouse were fixed in 4% paraformaldehyde and 4% sucrose overnight at 4 °C and then washed three times in phosphate-buffered saline solution (PBS). Cells then were permeabilized with 0.1% Triton X-100 in PBS for 15 min, followed by 30 min incubation at room temperature in blocking buffer (4% normal goat serum in PBS). Cells were incubated with antibody against Rac (1:10,000 dilution, Upstate Biotechnology, Lake Placid, NY) and synaptotagmin (1:1000 dilution, Santa Cruz, CA) for 2 h at room temperature and then rinsed three times with PBS. Cells then were incubated with a secondary antibody (CY3 or FITC, 1:500 dilution, Jackson ImmunoResearch Laboratories, Westgrove, PA) for 30 min, washed three times with PBS, mounted, and analyzed using a Zeiss LSM 510 META confocal microscope system (Zeiss, Oberkochen, Germany).
Western blot analysis
Equivalent amounts of protein were resolved via electrophoresis on 12% SDS–PAGE gels, transferred to polyvinylidene difluoride (PVDF) membranes, and incubated in TBS with 0.1% Tween 20 (TTBS) containing 5% non-fat milk for 1 h at room temperature. Blots were incubated with the corresponding primary antibody (Rac, NMDA-receptor subunit NR1, Na+/K+ ATPase, or PSD95 from Upstate Bio-technology; synaptotagmin or actin from Santa Cruz, and GST from Cell Signalling Technology, Beverly, MA) for 1 h at room temperature under shaking, washed three times for 10 min in TTBS, followed by incubation with a horseradish peroxidase-conjugated goat anti-mouse or rabbit IgG (1:5000 dilution, Promega, Madison, WI). Blots were visualized using enhanced chemiluminescence (ECL, Amersham Biosciences, Piscataway, NJ). The bands of each Western blot were quantified from film exposures in the linear range of each antibody and normalized to percentage of total Rac for each sample with densitometry using a desktop scanner and NIH image software to determine the amount of immunoreactivity.
Data analysis
Antibody binding to the antigen of interest in CA1 homogenates or fractions from control and drug-treated slices was expressed as a percent of antibody binding to the respective antigens in control slices. Statistical analysis was performed using either Student’s t tests for normally distributed variables to determine the significant differences between control and treatment groups or ANOVA followed by Dunnett’s comparison to control or Tukey multiple comparison post tests. A p value of less than 0.05 was considered statistically significant.
Results
Subcellular localization of Rac in the mouse brain and in the hippocampus
We first investigated the distribution of Rac in the mouse brain by examining expression levels in cortex, cerebellum, hippocampus, and brain stem. We found that in the mouse brain Rac was detected in the cortex, cerebellum, hippocampus, and brain stem as measured on Western blots of homogenates from each area (Fig. 1A).
Fig. 1.
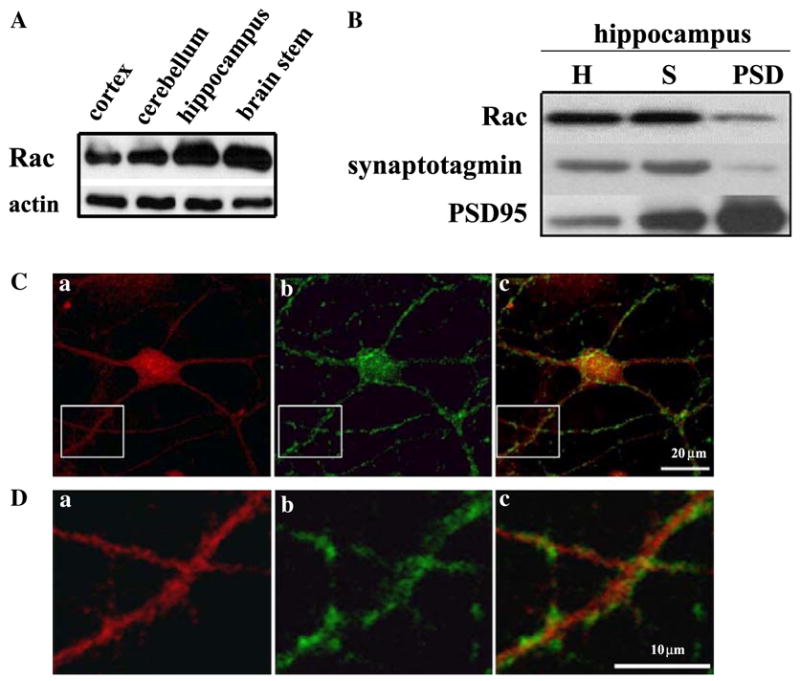
Expression of Rac in the mouse brain and hippocampus. (A) Western blots showing the distribution of Rac (10 μg protein/lane) in whole homogenates of cortex, cerebellum, hippocampus, and brain stem. Antibodies against actin (1:1000) were used to demonstrate equal protein loading, (B) synaptic localization of Rac in the mouse hippocampus. Mouse hippocampi were homogenized and fractionated as described in Materials and methods to obtain synaptoneurosomes and postsynaptic densities (PSDs). Equal amounts of protein (40 μg/lane) were separated on SDS–PAGE and probed on Western blots for Rac. Antibodies against synaptotagmin and PSD95 were used to demonstrate the purity of the synaptoneurosome and PSD preparations, respectively. Homogenates (H), synaptoneurosomes (S), and postsynaptic densities (PSD). (C,D) Co-localization of Rac with synaptotagmin in mouse hippocampal neurons. Cultured hippocampal neurons were prepared as described in Materials and methods. Representative confocal images were obtained from cultures that were double labeled using antibodies specific for Rac and the synaptic marker synaptotagmin. Labeling for Rac is indicated in red, synaptotagmin labeling is indicated in green, and dual labeling is indicated in yellow. Images were obtained with a 40× objective and optical zoom 3.0 (n = 4).
The localization and high expression of Rac found in the mouse hippocampus indicate that Rac could play a role in morphological changes that result in either dendrite formation or modification and establishment of new synapses, as reported in previous studies [9,20–23]. To investigate the subcellular localization of Rac in the mouse hippocampus, we further assessed the expression levels of Rac in homogenates, synaptoneurosomes, and postsynaptic density (PSD) preparations. We also used immunocytochemical techniques coupled with confocal microscopy to examine the cellular localization of Rac in mouse dissociated hippocampal cultures from area CA1/CA3. Our biochemical studies revealed that Rac was present in homogenates, synaptoneurosomes, and PSD preparations from hippocampus (Fig. 1B). We observed that immunostaining for Rac was most pronounced in the cell bodies, but also was present in the dendritic arbor (Fig. 1C, a). In addition, we found that Rac was associated with the synaptic marker synaptotagmin (Fig. 1D, c). This synaptic marker can be recognized by the brightly stained puncta outlining cell bodies and dendrites [24]. Higher magnification revealed some association of Rac with synaptotagmin at synaptic sites (Fig. 1D, c). Taken together these biochemical and immunocytochemical observations indicate that Rac is localized throughout hippocampal neurons, including cell bodies and dendrites, and likely at synaptic sites.
NMDA receptor stimulation regulates Rac translocation to the membrane in hippocampal area CA1
Because neural activity through NMDA receptors and/or AMPA receptors can trigger dendritic arbor growth, extension of axons, and formation of new synapses, and because Rac has been reported to promote these processes [25], we examined whether activation of glutamatergic receptors in adult hippocampal slices could trigger membrane association of Rac, one of the critical steps for its proper interaction with target proteins. We evaluated membrane translocation of Rac after adult mouse hippocampal slices were exposed to NMDA utilizing biochemical fractionation methods. We found that an acute application of NMDA (100 μM for 3 min) induced translocation of Rac to the membrane fraction in mouse hippocampal area CA1 (Fig. 2). To examine whether activation of either group I metabotropic glutamate (mGluR) receptors or voltage-gated calcium channels (VGCC) also causes translocation of Rac, we measured the relative levels of Rac in cytosolic and membrane fractions of hippocampal slices treated with the group I mGluR agonist DHPG (50 μM for 5 min) and KCl (90 mM for 3 min). We failed to detect translocation of Rac in hippocampal slices exposed to either DHPG or KCl (Fig. 2). Taken together, these data indicate that NMDA receptor activation, but neither group I mGluR nor VGCC activation, induces translocation of Rac to the membrane in the mouse hippocampal area CA1.
Fig. 2.
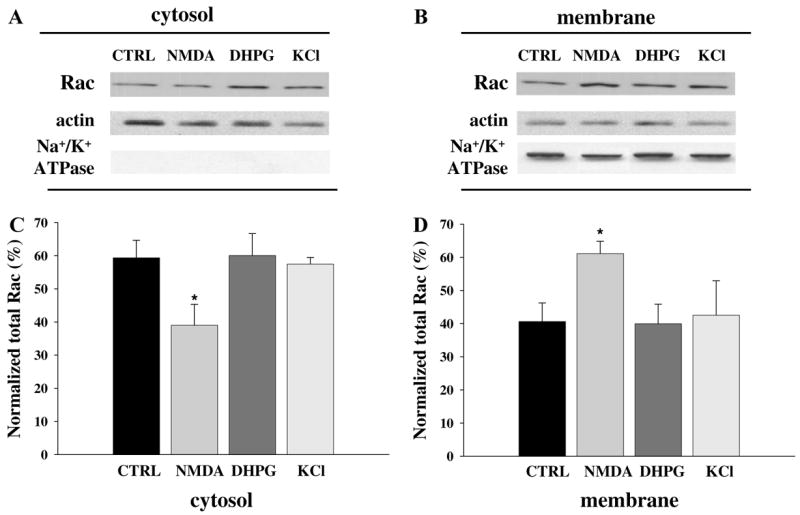
NMDA-induced membrane translocation of Rac in hippocampal slices. (A,B) Representative Western blots from hippocampal area CA1 fractions (10 μg protein/lane) showing the distribution of Rac in either cytosolic (A) or membrane (B) fractions after vehicle (CTRL), NMDA (100 μM for 3 min), DHPG (50 μM for 5 min), or KCl (90 mM for 5 min) treatment. Antibody against actin (1:1000) and Na+/K+ ATPase were used to demonstrate equal loading and the purity of the cytosolic and membrane fractions, respectively. (C,D) Group data (means ± SEM, n = 8) of Rac immunoreactivity in the cytosolic (C) and membrane (D) fractions of hippocampal area CA1 treated with vehicle ACSF (CTRL), NMDA (100 μM for 3 min), DHPG (50 μM for 5 min), or KCl (90 mM for 5 min). Rac immunoreactivity was normalized to % of total Rac for each sample. *Statistical significance (p < 0.05 by ANOVA).
NMDA receptor antagonists block the NMDA-induced translocation of Rac in hippocampal area CA1
To ensure that the NMDA receptor-dependent translocation of Rac in hippocampal slices was the result of NMDA receptor activation, we performed the NMDA treatment in the presence of two NMDA receptor antagonists, the glutamate site blocker D-(−)-2-amino-5-phospho-novaleric acid (APV) [26] and the channel blocker dizocilpine (MK801) [27]. Pre-incubation with either 50 μM APV or MK801 for 10 min blocked the NMDA-induced translocation of Rac in adult mouse hippocampal slices (Fig. 3). Moreover, we examined whether the NMDA-induced translocation of Rac was an effect of general membrane depolarization by exposing the hippocampal slices to NMDA in the presence of the Na+ channel blocker tetrodotoxin [28] (TTX 1 μM for 10 min). TTX had no effect on the NMDA-induced translocation of Rac (Fig. 3). Together, these findings indicate that the NMDA-induced change in Rac distribution from the cytosol to the membrane is via NMDA receptor activation and not general membrane depolarization.
Fig. 3.
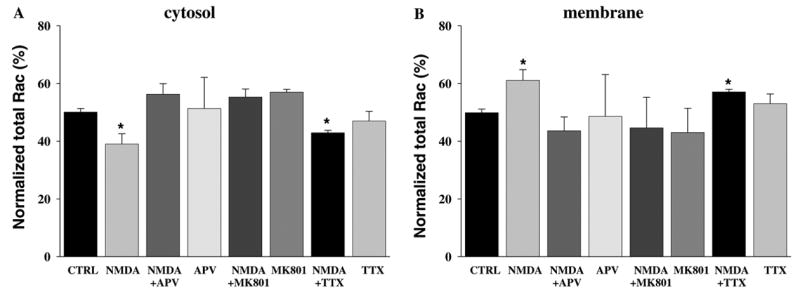
NMDA receptor activation triggers membrane translocation of Rac in hippocampal area CA1. Hippocampal slices were pre-incubated with either the NMDA receptor antagonists APV (50 μM) or MK801 (50 μM) for 10 min, or TTX (1 μM for 10 min) and then exposed to NMDA (100 μM for 3 min). (A,B) Group data (means ± SEM, n = 8) of Rac immunoreactivity in Western blots of hippocampal area CA1 cytosol (A) and membrane (B) fractions treated with vehicle ACSF (CTRL), or NMDA in the presence or absence of APV, MK801, or TTX. Rac immunoreactivity was normalized to % of total Rac for each sample. *Statistical significance (p < 0.05 by ANOVA).
NMDA receptor stimulation increases Rac levels in the postsynaptic densities in the mouse hippocampus
In an attempt to address whether the change in Rac distribution reported in the CA1 area after NMDA receptor activation occurred at synaptic sites, we isolated homogenate, synaptoneurosome, and PSD fractions from control (ACSF) and NMDA-treated samples, and probed these samples for Rac on Western blots. We found that an acute application of NMDA (100 μM for 3 min) induced an increase in Rac in the PSD fraction (Fig. 4). We observed no alterations in the level of Rac in either homogenate or synaptoneurosome fractions. These findings suggest that NMDA receptor activation increases the levels of Rac in the PSD fraction in hippocampal area CA1.
Fig. 4.
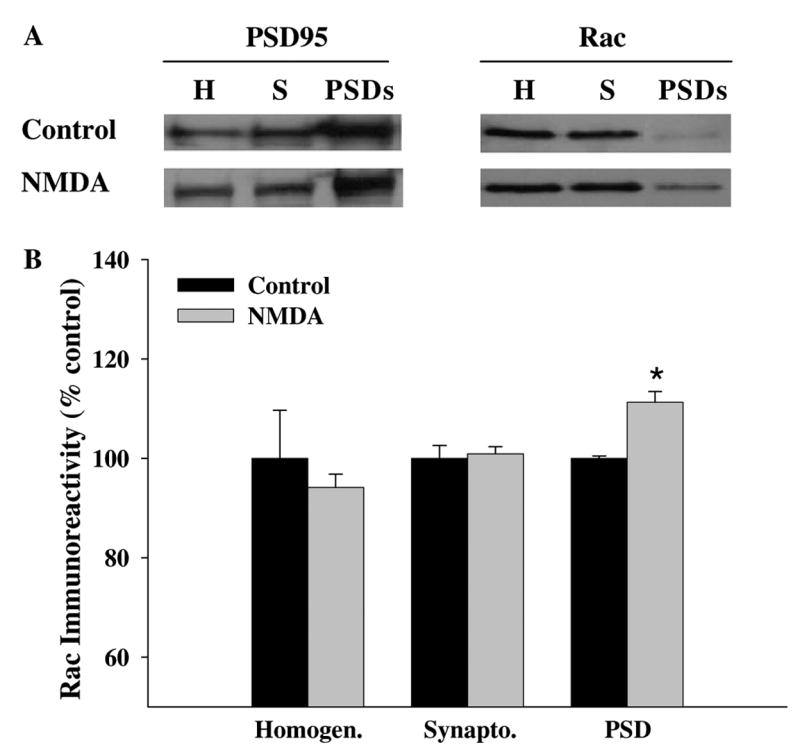
NMDA receptor activation increases Rac levels in PSDs. (A) Mouse hippocampi were homogenized and fractionated as described in Materials and methods to obtain synaptoneurosomes and postsynaptic densities (PSDs) after NMDA application (100 μM for 3 min). Equal amounts of protein (40 μg/lane) were separated on SDS–PAGE and probed on Western blots for Rac. An antibody against PSD95 was used to demonstrate the purity of the preparations. Representative blots for homogenates (H), synaptoneurosomes (S), and postsynaptic densities (PSD). (B) Group data (means ± SEM, n = 5) of Rac immunoreactivity in Western blots of mouse hippocampal slices treated with vehicle ACSF (Control) or NMDA. Rac immunoreactivity was normalized to % control for each sample. *Statistical significance (p < 0.05 by Student’s t test).
NMDA receptor activation triggers activation of Rac in hippocampal area CA1
Rac involvement in transmission of cellular signals depends not only on membrane-association, but also by shifting from an inactive GDP-bound state to an active GTP-bound state. Therefore, to determine whether the NMDA-induced changes in Rac membrane-association correlate with Rac activation, we treated hippocampal slices with NMDA and then performed an affinity purification assay to measure Rac-GTP. As shown in Fig. 5, activation of Rac was observed in mouse hippocampal slices after NMDA exposure. This activation was specific to the NMDA receptor activation because pre-incubation with either 50 μM APV or 50 μM MK801 prevented the NMDA-induced activation of Rac (Fig. 5). These data indicate that NMDA receptor activation triggers not only membrane translocation, but also activation of Rac in the mouse hippocampal area CA1.
Fig. 5.
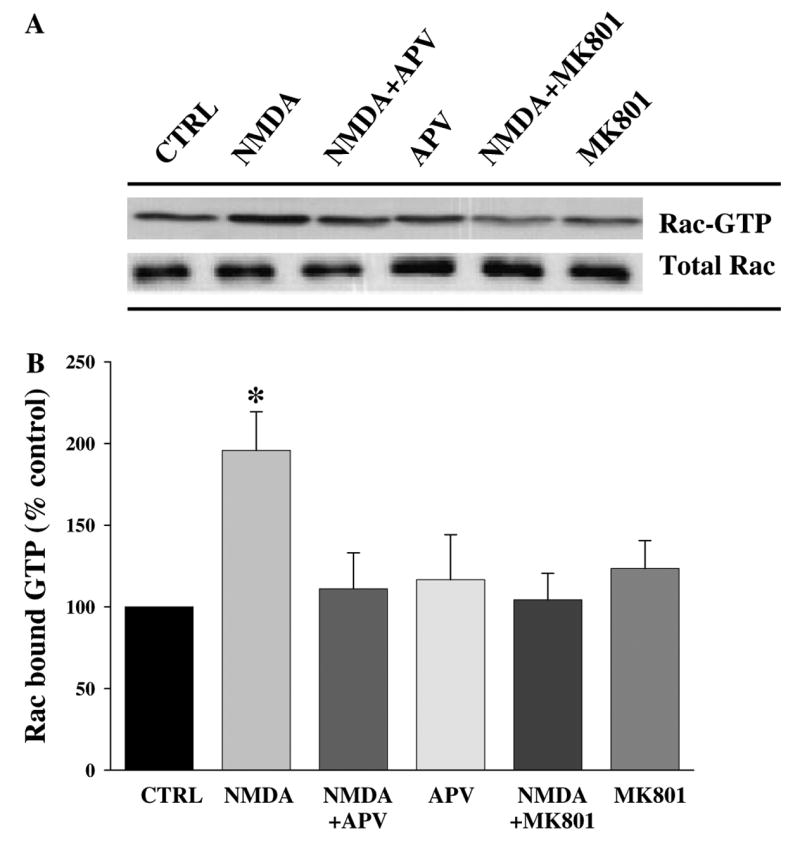
NMDA receptor activation triggers activation of Rac. Hippocampal slices were pre-incubated as described in Fig. 3. (A) Representative Western blot showing the activation of Rac after treatment of slices with NMDA and/or NMDA receptor antagonist. (B) Analysis of Rac activation (Rac-bound-GTP). Group data (means ± SEM, n = 6) of Rac-GTP in hippocampal slices treated with vehicle (CTRL), or NMDA in the presence or absence of APV, or MK801. Data were normalized relative to control samples and expressed as % of control. *Statistical significance (p < 0.05 by ANOVA).
NMDA-induced activation of Rac results in a physical association of Rac with the NMDA receptor complex
We next asked whether NMDA, in addition to triggering membrane translocation and activation of Rac, also caused a physical association of Rac with the NMDA receptor. We treated hippocampal slices with NMDA, followed by immunoprecipitation with Rac, NMDA-receptor subunit NR1, and probed the immunoprecipitates with either Rac- or NMDA receptor-specific antibody. We found that following NMDA exposure, there was an increase in the amount of Rac that co-immunoprecipitated with the NR1 subunit of the NMDA receptor (Fig. 6A). Moreover, there was an increase in the amount of the NR1 subunit that co-immunoprecipitated with Rac (Fig. 6B). Taken together, these results suggest that there is a native NMDA receptor complex consisting of both NR1 and NR2 subunits that when activated recruits Rac to form part of the receptor complex. To test whether NMDA receptor activation was indeed responsible for the association between Rac and NR1 subunit after NMDA application, we performed the NMDA treatment in the presence of APV and MK801. Pre-incubation with either 50 μM APV or MK801 for 10 min blocked the NMDA-induced association of Rac to the NR1 subunit of the NMDA receptor in adult mouse hippocampal slices (data not shown). Taken together, our data suggest that after NMDA stimulation Rac translocates to the membrane in close proximity to the NMDA receptor, existing in a complex with the NR1 subunit of the NMDA receptor.
Fig. 6.
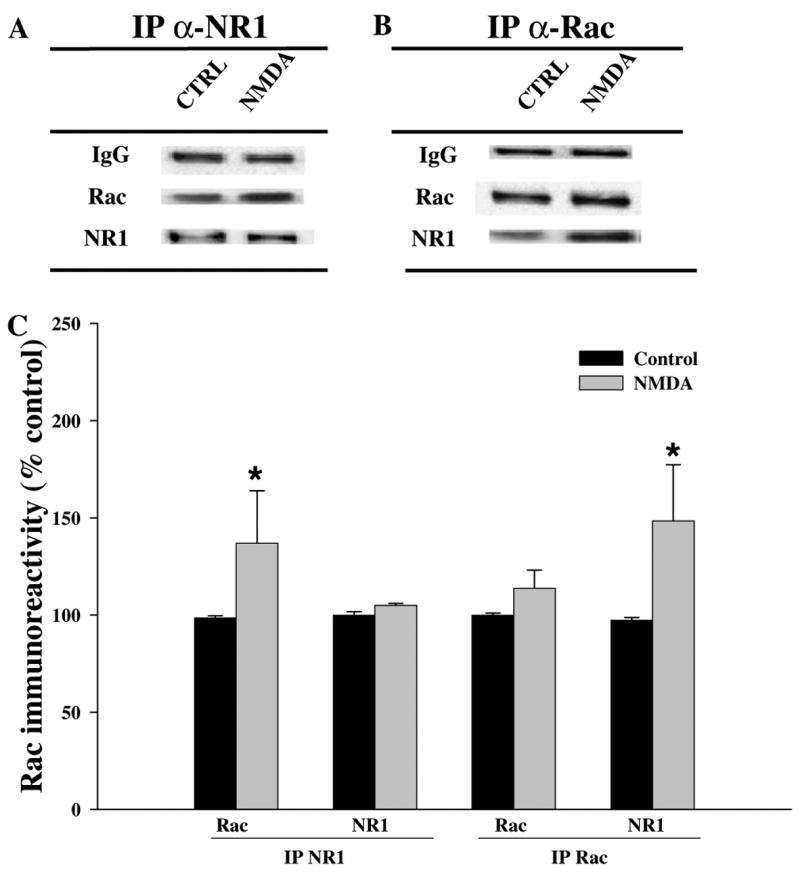
Interaction between Rac and NMDA receptor. Hippocampal slices were treated as described in Fig. 3. Homogenates were immunoprecipitated (IP) with antibodies recognizing (A) the C-terminus of NR1 and (B) Rac. The target epitope of the antibody against NR1 maps a region common to all NR1 isoforms, but only an additional band was detected at a lower concentration than the band expected. Approximately fifty percent of the immunoprecipitated samples were analyzed on Western blots for the presence of Rac and the NR1 subunit of the NMDA receptor. (C) Group data (means ± SEM, n = 5) of Rac and NR1 in hippocampal slices after IP. Data were normalized relative to control samples and expressed as % of control. *Statistical significance (p < 0.05 by Student’s t test).
Discussion
Dendritic outgrowth, new synapse formation, and synaptic plasticity in hippocampal neurons are of great interest because these processes are widely believed to provide the molecular and anatomical basis for memory [10,29–33]. The small GTPases of the Rho family have been reported to participate in the organization of the actin cytoskeleton and activation of signal transduction pathways that result in morphological changes that are believed to take place during synaptic plasticity in the brain [34–36]. In this study, we have provided evidence that one of these small GTPases, Rac, is present in the mouse hippocampus including synaptic sites (Fig. 1). We also found that NMDA receptor activation induced translocation of Rac to the membrane (Figs. 2 and 3) as well as activation of Rac (Fig. 5). Finally, we also demonstrated that NMDA receptor activation increased the association of Rac with the NR1 subunit of the NMDA receptor (Fig. 6). It is possible that the concentration of NMDA used in our study could cause neurotoxicity, as has been demonstrated in experiments with cultured neurons [37]. However, it was previously reported that hippocampal slices exhibit healthy electrophysiological responses after a brief exposure to 400 μM NMDA [37]. Further, this range of NMDA concentrations has been well documented for biochemical studies previously [38–40]. Thus, we believe our findings are consistent with the possibility that Rac participates in the morphological changes associated with NMDA receptor-dependent synaptic plasticity and memory formation in the adult mouse hippocampus, rather than changes associated with neurotoxicity.
In the rat CNS, GTPases from the Rho family, including Rac, have been shown to be expressed in hippocampal pyramidal neurons of CA1–CA4 regions, dentate granule cells, the neocortex, cerebellum, brain stem, and thalamus [41]. In rat hippocampal areas CA1 and CA3, Rac has been found to be present in stratum oriens and stratum radiatum [42]. Herein we found that Rac is widely distributed throughout the mouse brain with the highest levels being observed in the hippocampus (Fig. 1A).
We also examined the subcellular distribution of Rac in the adult mouse hippocampus. Immunoreactivity to Rac was found in synaptoneurosomes and PSDs, consistent with a synaptic presence of this protein (Fig. 1). Moreover, immunocytochemical examination of Rac in dissociated hippocampal cultures from area CA1/CA3 showed that Rac was present in cell bodies and was associated with the synaptic marker synaptotagmin, further indicating the presence of Rac at synapses. It is known that some GTPases of the Rho family (RhoA and RhoB) are activated by synaptic transmission in the CA1 region of hippocampus, via NMDA receptor stimulation [43]. Dendritic filopodia and spines undergo dynamic changes in response to synaptic activity, including stimulation shown to produce LTP [44–47]. Thus, this dendritic localization of Rac may reflect an important role in synaptic plasticity, whereas staining in cell bodies may reflect an important function in processes such as gene transcription. This idea would be consistent with recent studies showing that altering Rho family signaling usually leads to defects in several normal cellular functions [48]. These proteins may be activated at specific locations within the cell, in order to orchestrate morphological changes at these sites.
Recent studies have proposed a model for Rho GTPase-mediated dendritic arbor growth in which neuronal activity, via NMDA receptors, would activate Rac and decrease RhoA activation [10,25]. The dendritic spines of glutamatergic synapses are initially formed in response to NMDA receptor activation and synapses are subsequently stabilized by the expression of functional AMPA receptors [49]. It is generally accepted that in order to trigger a response, small GTPases have to be in the GTP conformation and associated to membranes. Our findings indicate that NMDA receptor activation increases Rac association to membrane as well as the amount of Rac bound to GTP in adult mouse hippocampal slices. The lack of Rac translocation following stimulation of group I mGluRs or VGCCs could be the result of those receptors/channels not being needed for the initial formation of synapses, or Rac not participating in the later stabilization of new synapses regulated by these types of receptors/channels. Translocation to the membrane fraction is controlled by the activation state of the GTPase [50]. For example, PDGF stimulates translocation of Rac1 in Swiss 3T3 fibroblasts [6,7] and stimulates GTP loading of Rac through activation of PI3K [51]. Whether PI3K is responsible for the NMDA receptor-dependent translocation and activation of Rac awaits further investigation.
A functional NMDA receptor complex consists of different proteins, including receptor subunits (NR1, NR2A–NR2D), adaptor proteins, signaling proteins, cytoskeletal proteins, and cell adhesion proteins [52]. Rac has been reported to be part of this complex as determined by proteomic analysis of the NMDA receptor complex [52]. NR1 subunits together with either NR2A or NR2B make up the type of NMDA receptors that contribute to synaptic responses in the hippocampus [53]. The NR2A and NR2B subunits contribute to LTP induction in area CA1 [54]. Both NR2A and NR2B subunits activate signaling pathways which lead to LTP; in young animals both subunits contribute to LTP [55], whereas in adult animals NR2A-mediated signaling dominates [56]. Herein, our immunoprecipitation experiments using either antibodies to Rac or antibodies to NR1 indicate that Rac is assembled in NMDA receptor complexes, as reported previously [52]. We were able to detect association of Rac with the obligatory NR1 subunit of the NMDA receptor in immunoprecipitates obtained with either anti-NR1 or anti-Rac. The native functional NMDA receptor channel complex consists of both NR1 and NR2 subunits [57] and these subunits co-immunoprecipitate together [58]. In our study, the NR1 immunoprecipitate pulled-down Rac as shown by immunoblotting (Fig. 6B), suggesting an interaction between them. Specificity was further suggested by the reverse experiment in which the antibody against Rac also precipitated the NR1 NMDA-receptor subunit (Fig. 6A). It has been suggested that NMDA receptor activation that promotes events for dendritic growth and spine morphology through small G proteins proceeds by stimulating first other proteins such as GEFs, which in turn activate Rac [13]. We suspect that after NMDA receptor activation, Rac likely translocates in proximity to the receptor via interaction with other proteins in the NMDA receptor complex. This idea remains to be examined.
Regulation of small GTPase signaling appears to play a key role in neural cognitive function. At least three proteins (oligophrenin 1, aPIX, and p21-activated kinase [PAK]) interact directly with Rho GTPases and have been reported to be mutated in patients with non-syndromic mental retardation [59–63]. Thus, understanding how GTPases, including Rac, are normally regulated in the adult hippocampus will provide important information regarding the signaling mechanisms that underlie synaptic plasticity and learning and memory as well as insights into the basis of diseases involving memory impairment, such as non-syndromic X-linked mental retardation (MRX).
Acknowledgments
This work was supported by NIH Grants NS048037 (to M.V.T) and NS34007 (to E.K.).
References
- 1.Foreman J, Demidchik V, Bothwell HHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Browniess C, Jones JDG, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 2.Smith SJ, Rittinger K. Preparation of GTPases for structural and biophysical analyses. Methods Mol Biol. 2002;189:13–24. doi: 10.1385/1-59259-281-3:013. [DOI] [PubMed] [Google Scholar]
- 3.Harwood A, Braga VMM. Cdc42 and GSK-3: signals at the crossroads. Nat Cell Biol. 2003;5:275–277. doi: 10.1038/ncb0403-275. [DOI] [PubMed] [Google Scholar]
- 4.Kim BC, Yi JY, Yi SJ, Shin IC, Ha KS, Jhon BH. Rac GTPase activity is essential for EGF-induced mitogenesis. Mol Cells. 1998;8:90–95. [PubMed] [Google Scholar]
- 5.Welsh CF. Rho GTPases as key transducers of proliferative signals in G1 cell cycle regulation. Breast Cancer Res Treat. 2004;84:33–42. doi: 10.1023/B:BREA.0000018425.31633.07. [DOI] [PubMed] [Google Scholar]
- 6.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 7.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 9.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. Rac1 and Cdc42 but not RhoA or Rho Kinase activities are required for neurite outgrowth induced by the netrin-1 receptor DCC (Deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem. 2002;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- 11.O’Kane EM, Stone TW, Morris BJ. Increased long-term potentiation in the CA1 region of rat hippocampus via modulation of GTPase signalling or inhibition of Rho kinase. Neuropharmacology. 2004;46:879–887. doi: 10.1016/j.neuropharm.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Leemhuis J, Mayer U, Barth H, Schmidt G, Meyer DK. The small GTPase Rac is involved in clustering of hippocampal neurons and fasciculation of their neurites. Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;370:211–222. doi: 10.1007/s00210-004-0965-y. [DOI] [PubMed] [Google Scholar]
- 13.Tolias KF, Biko3 JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Webb DJ, Asmussen H, Niu S, Horwitz AF. A GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J Neurosci. 2005;25:3379–3388. doi: 10.1523/JNEUROSCI.3553-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro P, Flatau G, Doye A, Boyer L, Oregioni O, Mege JL, Landraud L, Lemichez E. Activation and proteasomal degradation of Rho GTPases by cytotoxic necrotizing factor-1 elicit a controlled inflammatory response. J Biol Chem. 2004;279:35849–35857. doi: 10.1074/jbc.M401580200. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 19.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 20.Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 21.Mikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 22.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 23.Caron E. Rac signalling: a radical view. Nat Cell Biol. 2003;5:185–187. doi: 10.1038/ncb0303-185. [DOI] [PubMed] [Google Scholar]
- 24.Caceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Aizenman CD, Cline HT. Regulation of Rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 26.Davies J, Watkins JC. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982;235:378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- 27.Wong EHF, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK801 is a potent NMDA antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleitz J, tosch C, Beile A, Peters T. The protective action of tetrodotoxin and (±)Kavain on anaerobic glycolysis, ATP content and intracellular NA+ and Ca2+ of anoxic brain vesicles. Neuropharmacology. 1996;35:1743–1752. doi: 10.1016/s0028-3908(96)00106-2. [DOI] [PubMed] [Google Scholar]
- 29.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 30.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 32.Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- 33.Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- 34.Mackay DJG, Nobes CD, Hall A. The Rho’s progress: a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. [DOI] [PubMed] [Google Scholar]
- 35.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chetkovich DM, Gray R, Johnston D, Sweatt JD. N-Methyl-D-aspartate receptor activation increases cAMP levels and voltage-gated Ca2+ channel activity in area CA1 of hippocampus. Proc Natl Acad Sci USA. 1991;88:6467–6471. doi: 10.1073/pnas.88.15.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem. 2004;91:462–470. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- 39.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 40.Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olenik C, Barth H, Just I, Aktories K, Meyer DK. Gene expression of the small GTP-binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult rat brain. Mol Brain Res. 1997;52:263–269. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 42.O’Kane EM, Stone TW, Morris BJ. Distribution of Rho family GTPases in the adult rat hippocampus and cerebellum. Mol Brain Res. 2003;114:1–8. doi: 10.1016/s0169-328x(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 43.O’Kane EM, Stone TW, Morris BJ. Activation of Rho GTPases by synaptic transmission in the hippocampus. J Neurochem. 2003;87:1309–1312. doi: 10.1046/j.1471-4159.2003.02102.x. [DOI] [PubMed] [Google Scholar]
- 44.Buchs PA, Muller D. Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc Natl Acad Sci USA. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 46.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 47.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Webb DJ, Asmussen HA, Horwitz AF. Synapse formation is regulated by the signaling adaptor GIT1. J Cell Biol. 2003;161:131–138. doi: 10.1083/jcb.200211002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher JL, Dani JA. Nicotinic receptors on hippocampal cultures can increase synaptic glutamate currents while decreasing the NMDA-receptor component. Neuropharmacology. 2000;39:2756–2769. doi: 10.1016/s0028-3908(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 50.Fleming IN, Elliott CM, Exton JH. Differential translocation of Rho family GTPases by lysophosphatidic acid, endothelin-1, and platelet-derived growth factor. J Biol Chem. 1996;271:33067–33073. doi: 10.1074/jbc.271.51.33067. [DOI] [PubMed] [Google Scholar]
- 51.Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evan T, Symons M. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 52.Husi H, Grant GN. Proteomics of the nervous system. Trends Neurosci. 2001;24:259–266. doi: 10.1016/s0166-2236(00)01792-6. [DOI] [PubMed] [Google Scholar]
- 53.Kirson ED, Yaari Y. Synaptic NMDA receptors in developing mouse hippocampal neurons: functional properties and sensibility to ifenprodil. J Physiol. 1996;497:437–455. doi: 10.1113/jphysiol.1996.sp021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohr G, Jensen V, Koester HJ, Mihaljevic ALA, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby O. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frost JA, Xu S, Hutchison MR, Marcus S, Cobb MH. Actions of Rho family small G proteins and p21-activated protein kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sans N, Petralia RS, Wang YX, Blahos J, II, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sessoms JS, Chen SJ, Chetkovich DM, Powell CM, Roberson ED, Sweatt JD, Klann E. Ca(2+)-induced persistent protein kinase C activation in rat hippocampal homogenates. Second Messengers Phosphoproteins. 1992;14:109–126. [PubMed] [Google Scholar]
- 58.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 59.Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- 60.Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Crollius HR, Carrie A, Fauchereau F, Cherry M, Briault S, Hamel B, Fryns JP, Beldjord C, Kahn A, Moraine C, Chelly J. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–926. doi: 10.1038/31940. [DOI] [PubMed] [Google Scholar]
- 61.Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, Boavida MG, David D, Chelly J, Fryns JP, Moraine C, Ropers HH, Hamel BCJ, van Bokhoven H, Gal A. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet. 2000;26:247–250. doi: 10.1038/80002. [DOI] [PubMed] [Google Scholar]
- 62.Barnes AP, Milgram SL. Signals from the X: signal transduction and X-linked mental retardation. Int J Dev Neurosci. 2002;20:397–406. doi: 10.1016/s0736-5748(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 63.Ramakers GJA. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]


