Abstract
Superoxide has been shown to be critical for hippocampal long-term potentiation (LTP) and hippocampus-dependent memory function. A possible source for the generation of superoxide during these processes is NADPH oxidase. The active oxidase consists of two membrane proteins, gp91phox and p22phox, and four cytosolic proteins, p40phox, p47phox, p67phox, and Rac. Upon stimulation, the cytosolic proteins translocate to the membrane to form a complex with the membrane components, which results in production of superoxide. Here, we determined the presence, localization, and functionality of a NADPH oxidase in mouse hippocampus by examining the NADPH oxidase proteins as well as the production of superoxide. All of the NADPH oxidase proteins were present in hippocampal homogenates and enriched in synaptoneurosome preparations. Immunocytochemical analysis of cultured hippocampal neurons indicated that all NADPH oxidase proteins were localized in neuronal cell bodies as well as dendrites. Furthermore, double labeling analysis using antibodies to p67phox and the presynaptic marker synaptophysin suggest a close association of the NADPH oxidase subunits with synaptic sites. Finally, stimulation of hippocampal slices with phorbol esters triggered translocation of the cytoplasmic NADPH oxidase proteins to the membrane and an increase in superoxide production that was blocked by inhibitors of NADPH oxidase. Taken together, our data suggest that NADPH oxidase is present in mouse hippocampus and might be the source of superoxide production required for LTP and memory function.
Introduction
NADPH oxidase is a unique, multi-protein, electron transport system that produces large quantities of superoxide via the reduction of molecular oxygen (Babior, 2004; Cross and Segal, 2004; Shatwell and Segal, 1996). The active oxidase consists of a membrane-bound flavocytochrome b558 that carries a noncovalently bound FAD cofactor and two heme groups. Cytochrome b558, which consists of two membrane proteins (gp91phox and p22phox), takes electrons from NADPH and passes them, via FAD and heme, to molecular oxygen. Activation of the electron transport chain requires at least three cytosolic proteins; p47phox, p67phox, and the small GTP-binding protein Rac. Upon stimulation, the cytosolic proteins translocate to the membrane to form a complex with cytochrome b558, which results in production of superoxide (Heyworth et al., 1991; Park et al., 1992; Quinn and Gauss, 2004). Both p47phox and p67phox contain two Src homology 3 (SH3) domains, as does p40phox, a cytosolic protein that associates with p67phox (Wientjes et al., 1993). Although p40phox translocates to the membrane upon cell stimulation (Wientjes et al., 1993), it is not required for activation of the oxidase (Bokoch and Diebold, 2002; de Mendez and Leto, 1995).
NADPH oxidase has been thoroughly studied in phagocytic cells (Babior, 2004; Batot et al., 1995; Cross and Segal, 2004); however, the presence of analogous NADPH oxidases in non-phagocytic cells also has been described (Bokoch and Knaus, 2003; Bolscher et al., 1990; Brozna et al., 1988; Lambeth, 2004). NADPH oxidase-like enzymes have been identified in vascular smooth muscle cells (Fukui et al., 1995), fibroblasts (Thannickal and Fanburg, 1995), endothelial cells (Jones et al., 1996), rat peripheral neurons (Dvorakova et al., 1999; Tammariello et al., 2000), cerebral cortical neurons (Noh and Koh, 2000), hippocampal pyramidal neurons, and cerebellar Purkinje neurons (Mizuki et al., 1998; Serrano et al., 2003). In addition, homologs of the cytochrome b558 component gp91phox have been found in several tissues (Babior et al., 2002; Lambeth, 2004).
In non-phagocytic cells, NADPH oxidase generates reactive oxygen species (ROS) that are involved in regulation of many cellular activities such as transcription, intracellular signaling, and host defense (Babior, 1999; Finkel, 1999; Lambeth, 2004). Moreover, it has been suggested that NADPH oxidase might be the source of superoxide required for hippocampal long-term potentiation (LTP) and hippocampus-dependent memory (Knapp and Klann, 2002; Thiels et al., 2000). In this manuscript, we report the expression of synaptically localized NADPH oxidase protein complex in the mouse hippocampus. Additional experiments revealed that cytosolic proteins translocate to the membrane upon stimulation with a phorbol ester, and that this translocation correlates with an increase in superoxide production in hippocampal slices. These findings are consistent with the idea that a neuronal NADPH oxidase is the enzymatic source of superoxide that is required for LTP and memory in the hippocampus.
Results
NADPH oxidase proteins are present in the mouse brain in the appropriate subcellular fraction
To determine whether NADPH oxidase is present in the mouse brain, we examined the protein levels of NADPH oxidase subunits in cortex, cerebellum, hippocampus, and brain stem of mice using a panel of monoclonal and polyclonal antibodies raised against human NADPH oxidase subunits. Each of the NADPH oxidase subunits was detected in all the brain areas examined as measured on Western blots of homogenates from each area (Fig. 1A). Of all the brain regions, the hippocampus exhibited the strongest immunoreactivity. In addition, there were differences in the immunoreactivity of the proteins in all the brain areas examined, with p67phox and gp91phox subunits exhibiting higher levels of immunoreactivity than p47phox, p40phox, and p22phox. These findings indicate that NADPH oxidase proteins are distributed in several brain tissues and are consistent with immunocytochemical analysis of NADPH oxidase proteins in the mouse brain (Serrano et al., 2003).
Fig. 1.
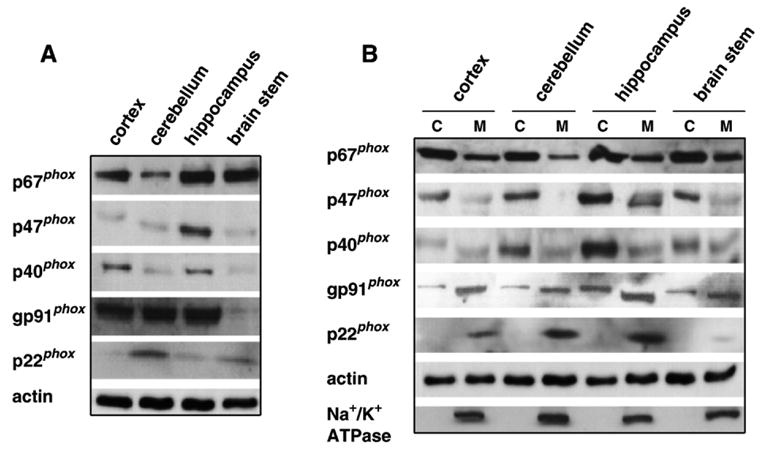
Expression of the NADPH oxidase enzyme complex in the mouse brain. (A) Distribution of phox proteins (p67phox, p47phox, p40phox, gp91phox, and p22phox, 10 μg protein/lane) in whole homogenates of the cortex, cerebellum, hippocampus, and brain stem. (B) Distribution of phox proteins (p67phox, p47phox, p40phox, gp91phox, and p22phox, 40 μg protein/lane) in cytosolic (C) or membrane (M) fractions of cortex, cerebellum, hippocampus, and brain stem homogenates. Antibodies against actin and Na+/K+ ATPase were used to demonstrate equal protein loading and the purity of the cytosolic and membrane fractions, respectively.
To establish whether the subcellular localization of the NADPH oxidase proteins in mouse brain resembles that observed in phagocytes, we examined the localization of the NADPH oxidase proteins in both cytosolic and membrane fractions. Protein homogenates from cortex, cerebellum, hippocampus, and brain stem were centrifuged to obtain cytosolic and membrane fractions and probed on Western blots with antibodies for each of the proteins. The total levels of cytosolic subunits, p67phox, p47phox, and p40phox were noticeably higher in the cytosolic fraction as compared to the membrane fraction in all areas of the brain (Fig. 1B). Conversely, the membrane-bound subunits, gp91phox and p22phox, were enriched in the membrane fraction as compared to the cytosolic fraction (Fig. 1B). The electrophoretic mobility of the large subunit gp91phox detected in the membrane fraction was identical to that of gp91phox in mouse phagocytes (data not shown). However, we detected small amounts of this subunit in the cytosolic fraction that migrated slower on SDS-PAGE, suggesting the possibility of additional glycosylated groups that might be lost once this subunit is anchored to the membrane. These results are consistent with previous studies showing that the NADPH oxidase is present in the brain (Dvorakova et al., 1999; Mizuki et al., 1998; Noh and Koh, 2000; Tammariello et al., 2000).
Immunocytochemical localization of NADPH oxidase proteins in hippocampal mouse cultures
To further investigate the cellular localization of NADPH oxidase in neurons in the mouse brain, we examined dissociated hippocampal neuronal cultures from area CA1/CA3 utilizing immunocytochemistry coupled with confocal microscopy. Morphologically, dendrites are thick at the base and narrow with increasing distance from the cell body, whereas axonal processes are long, thin, and fairly uniform in diameter (Caceres et al., 1986). Fig. 2 shows the immunocytochemical localization of gp91phox, p22phox, p67phox, and p47phox in mouse hippocampal neurons. Immunostaining for all of the subunits was most pronounced in the cell bodies, but the proteins also were present in the dendritic arbor. The membrane subunits gp91phox and p22phox also were present in the axonal arbor. These findings indicate that the NADPH oxidase is localized throughout hippocampal neurons, including cell bodies, dendrites, and axons.
Fig. 2.
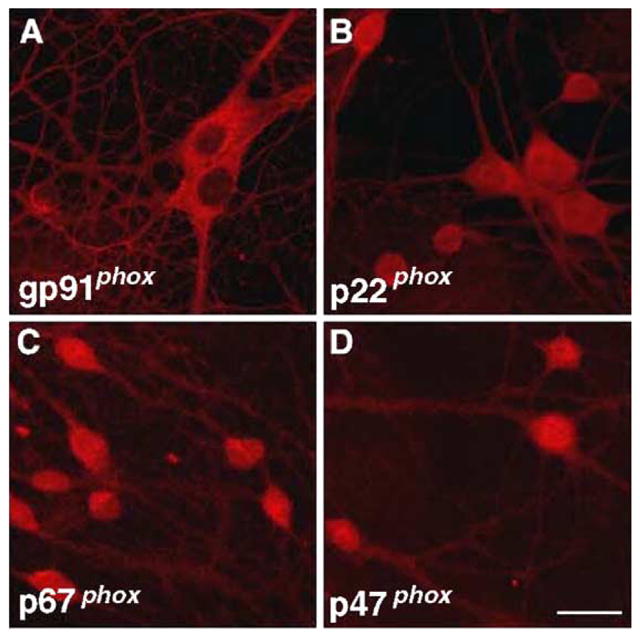
Immunocytochemical localization of NADPH oxidase proteins in hippocampal neurons. Cultured hippocampal neurons were prepared as described in Experimental methods. Representative confocal images were obtained from cultures that were labeled using antibodies specific for (A) gp91phox, (B) p22phox, (C) p67phox, and (D) p47phox. Images were obtained with a 40× objective. Scale bar 20 μm.
Fractionation of hippocampal homogenates suggests synaptic localization of NADPH oxidase proteins
To further investigate the subcellular localization of the NADPH oxidase complex in the mouse brain, we assessed the expression levels of the oxidase subunits in homogenates, synaptoneurosome, and postsynaptic density (PSD) preparations from whole brain as well as the hippocampus. Both cytosolic (p67phox) and membrane (gp91phox) components of NADPH oxidase were present in homogenates, synaptoneurosomes, and PSD preparations from whole brain (Fig. 3A) and hippocampus (Fig. 3B). We also examined the synaptic localization of the NADPH oxidase subunits in mouse hippocampal cultures using immunocytochemistry. We observed that the NADPH oxidase subunits p67phox and gp91phox in several instances were closely associated with the synaptic markers synaptophysin and synaptotagmin, respectively (Fig. 3C). These synaptic markers can be recognized by their brightly stained puncta outlining dendrites and cell bodies. Staining for p67phox was localized in the cytoplasm and dendrites, but higher magnification revealed a more punctate staining in some areas that was associated with synaptophysin staining (Fig. 3C, upper panels). Moreover, we observed immunoreactivity for gp91phox in cell bodies, dendrites, and axons (Fig. 3C, lower panels). Similar results were observed with p47phox and p22phox (data not shown). Taken together, these biochemical and immunocytochemical observations indicate that NADPH oxidase is present in hippocampal neurons, including synaptic sites.
Fig. 3.
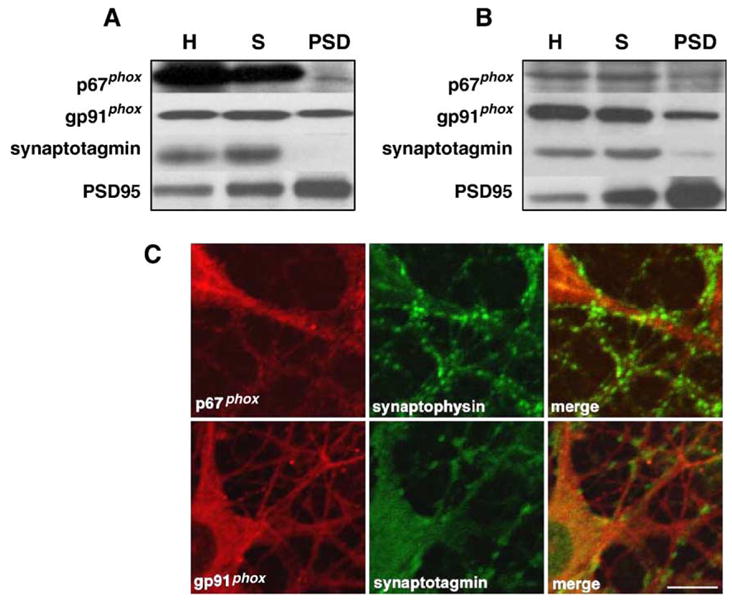
Synaptic localization of NADPH oxidase proteins in the mouse hippocampus. Either (A) whole brain or (B) hippocampus were homogenized and fractionated as described in Experimental methods to obtain synaptoneurosomes and postsynaptic densities. Shown are Western blots for representative cytosolic (p67phox) and membrane (gp91phox) components of NADPH oxidase. Antibodies against synaptotagmin and PSD95 were used to demonstrate the purity of the synaptoneurosome and PSD preparations. Homogenates (H), synaptoneurosomes (S), postsynaptic densities (PSD). C) Co-localization of p67phox and gp91phox with synaptophysin and synaptotagmin in mouse hippocampal neurons. Cultured hippocampal neurons were prepared as described in Experimental methods. Representative confocal images were obtained from cultures that were double labeled using antibodies specific for either p67phox or gp91phox, and the synaptic markers synaptophysin and synaptotagmin, respectively. Labeling for p67phox and gp91phox is indicated in red, synaptophysin and synaptotagmin labeling is indicated by green, and dual labeling is indicated by yellow. Images were obtained with a 40× objective and optical zoom 3.0. Scale bar 10 μm.
Phorbol ester-induced membrane translocation of cytosolic NADPH oxidase components
Phorbol esters have been shown to stimulate NADPH oxidase through activation of protein kinase C in phagocytes (Lu et al., 1993; Quinn et al., 1993). Therefore, using immunocytochemistry, we first examined whether treatment of hippocampal cultures with the phorbol ester phorbol-12-myristate-13-acetate (PMA) could induce changes in the distribution of the cytosolic subunits that would suggest activation of the NADPH oxidase. Fig. 4 shows a high magnification image of dendrites of hippocampal cultures stained with antibodies for p67phox and synaptophysin after the cultures were treated with PMA. The staining pattern for p67phox after treatment with PMA appeared to be more punctate compared to untreated cultures. The PMA-induced increases in punctate p67phox staining also was more closely associated with synaptophysin as indicated by an increase in double labeling of the neurons (Fig. 4, bottom panels). In addition, the amount of synaptophysin associated with synaptic sites also increased after PMA stimulation, consistent with a previous report (Chapell and Rosenberg, 1996). Similar findings were observed in experiments using an antibody against p47phox (data not shown). These findings suggest that PMA causes membrane translocation of cytosolic NADPH oxidase proteins to synapses in hippocampal neurons.
Fig. 4.

PMA-induced co-localization of p67phox and synaptophysin in mouse hippocampal neurons. Cultured hippocampal neurons were prepared as described in Experimental methods. Hippocampal neurons were incubated in media either with or without 1 μM PMA for 10 min. Representative confocal images were obtained from cultures that were double labeled using antibodies specific for p67phox and synaptophysin. Labeling for p67phox is indicated in red, synaptophysin labeling is indicated by green, and dual labeling is indicated by yellow. Similar results were obtained in four separate experiments. Images were obtained with a 40× objective and optical zoom 3.0. Scale bar 10 μm.
To more directly determine whether PMA stimulates NADPH oxidase activity in the hippocampus, we evaluated membrane translocation of cytosolic NADPH oxidase proteins in hippocampal slices utilizing biochemical fractionation methods. Treatment of hippocampal slices with PMA dramatically increased the translocation of the cytosolic proteins (p40phox, p47phox, p67phox, and Rac) to the membrane compartment (Fig. 5). Taken together, the results of our immunocytochemical and biochemical studies indicate that in the mouse hippocampus, phorbol esters trigger the membrane translocation of cytosolic NADPH oxidase components in a manner similar to that observed in phagocytic cells.
Fig. 5.
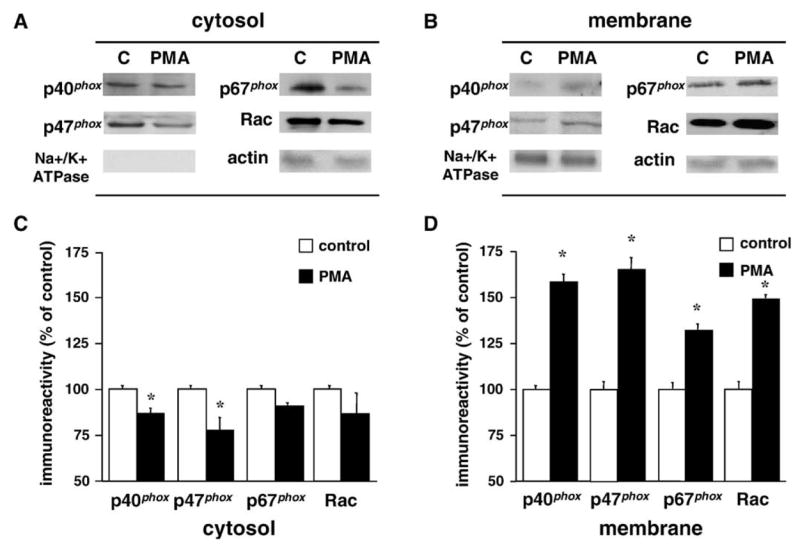
PMA-induced membrane translocation of cytosolic NADPH oxidase proteins in hippocampal slices. (A, B) Representative Western blots from hippocampal slice fractions (20 μg protein/lane) showing the distribution of cytosolic NADPH oxidase proteins in either cytosolic (A) or membrane (B) fractions after vehicle (C) or 1 μM PMA treatment for 5 min. Antibodies against actin and Na+/K+ ATPase were used to demonstrate equal protein loading and the purity of the cytosolic and membrane fractions, respectively. (C, D) Group data (mean ± SEM, n = 6) of NADPH oxidase cytosolic proteins immunoreactivity in the cytosolic (C) and membrane (D) fractions of hippocampal slices treated with vehicle (control, open bars) or 1 μM PMA treatment for 5 min (filled bars). Data were normalized relative to control samples in each fraction and expressed as % of control. *denotes statistical significance (P < 0.05 by Student’s t test).
Phorbol esters induced intracellular superoxide production in hippocampal slices
We proceeded to examine whether translocation of the cytosolic components of the NADPH oxidase induced by PMA resulted in the functional activation of the NADPH oxidase by measuring the production of superoxide in hippocampal slices using the nitroblue tetrazolium (NBT) assay. We found that treatment of hippocampal slices with PMA caused a statistically significant increase in superoxide production (Fig. 6). The PMA-induced increase in superoxide production was significantly reduced when slices were pretreated with either diphenylene iodonium (DPI; Fig. 6A), which inactivates the gp91phox subunit (Hancock and Jones, 1987) or 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF; Fig. 6B), which blocks the gp91phox-p47phox/p67phox interaction (Diatchuk et al., 1997). These findings are consistent with the idea that a functional NADPH oxidase is present in the mouse hippocampus.
Fig. 6.
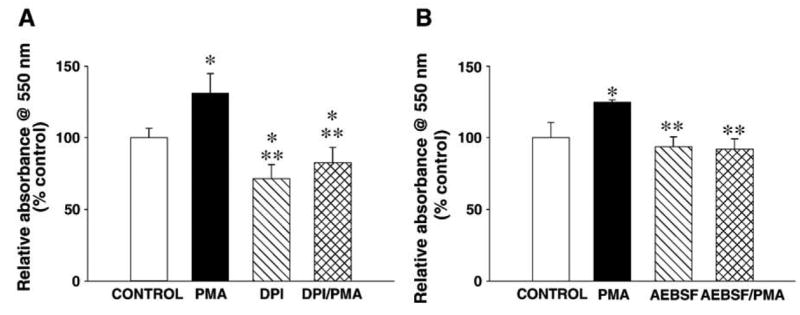
PMA-induced increases in superoxide in hippocampal slices are blocked by NADPH oxidase inhibitors. Hippocampal slices were preincubated with either (A) DPI (10 μM for 10 min) or (B) AEBSF (10 μM for 1 h), treated with NBT (1 mg/ml for 3 min), and then treated with either PMA (2.4 μM) or vehicle (control) for 7 min. Slices then were prepared as described in Experimental methods and absorbance was measured at 550 nm. Group data (mean ± SEM, n = 5) was normalized relative to control samples and expressed as % of control. *denotes statistical significance respect to control (untreated slices), **denotes statistical significance respect to PMA-treated slices ( P < 0.001 by Bonferonni’s multiple comparison test).
Discussion
In this manuscript, we have characterized the presence of a functional NADPH oxidase or NADPH oxidase-like enzyme in the mouse brain. NADPH oxidase has been characterized extensively in phagocytic cells such as eosinophiles and neutrophils (Babior et al., 2002). Furthermore, it has been shown that NADPH oxidase proteins are expressed in microglia (Della Bianca et al., 1999; Shimohama et al., 2000). However, recent studies have shown that NADPH oxidase proteins also are present in the nervous system. Small amounts of p40phox and p67phox mRNA have been detected in the mouse brain via Northern blot analysis (Mizuki et al., 1998) and in situ hybridization studies revealed that p40phox mRNA was expressed in pyramidal neurons in areas CA1 and CA3 of the hippocampus, as well as granule cells of the dentate gyrus (Mizuki et al., 1998). In addition, p40phox mRNA was observed in both Purkinje cells and granule cells in the cerebellum (Mizuki et al., 1998). Subsequently, it was shown that gp91phox is present in rat sensory neurons (Dvorakova et al., 1999) and that several of the NADPH oxidase proteins are expressed in both sympathetic (Tammariello et al., 2000) and cortical neurons (Noh and Koh, 2000).
In this study, we expanded our previous observations on the distribution of the NADPH oxidase in the mouse brain (Serrano et al., 2003) by examining the protein levels and subcellular localization of NADPH oxidase subunits in the cortex, cerebellum, brain stem, and hippocampus. The subcellular localization of the NADPH oxidase subunits in the mouse brain resembles that observed in phagocytes at resting state, with gp91phox and p22phox located predominantly in the membrane, and p67phox, p47phox, and p40phox present in the cytosol (Fig. 1B). Immunocytochemical examination of the NADPH oxidase subunits in dissociated hippocampal cultures from area CA1/CA3 indicates the presence of these subunits throughout neurons, including cell bodies and dendrites. It should be noted that the expression of these subunits in astrocytes or microglia was not examined in this study. Although we observed immunoreactivity for all the NADPH oxidase subunits in the cell bodies and dendrites, axonal staining was only evident when antibodies against the membrane subunits were used, in particular gp91phox (Fig. 2). However, this difference in membrane versus cytoplasmic localization of the different NADPH oxidase proteins could be due to the ability of the antibodies to detect their antigens in immunocytochemical studies. Overall, our data indicate that the different NADPH oxidase subunits are found throughout the neuron, suggesting that NADPH oxidase could play multiple roles in neuronal signaling processes. For instance, the dendritic localization of NADPH oxidase may reflect an important role for the enzyme in signaling at the synapse during synaptic plasticity (Knapp and Klann, 2002; Thiels et al., 2000), whereas staining in neuronal cell bodies may reflect important functions for NADPH oxidase in additional non-synaptic signaling processes, including regulation of transcription factors such as NF-κB (Sadikot et al., 2004). Studies from a number of laboratories have suggested that ROS, including superoxide, may act as cellular messengers modulating the activity of protein kinases such as protein kinase C (PKC), mitogen-activated kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), and protein tyrosine kinases (PTK) signaling cascades (Klann and Thiels, 1999), each of which has been shown to play a role in synaptic plasticity. Our results indicate that superoxide produced by a functional NADPH oxidase could be acting as a source for superoxide in synaptic plasticity and other physiological processes in hippocampal neurons.
In this report, we also analyzed the subcellular localization of NADPH oxidase by analyzing p67phox, a cytosolic subunit, and gp91phox, a membrane subunit. We found that both proteins were enriched in synaptoneurosomes and both proteins were present in PSDs, consistent with a synaptic localization of this enzyme (Figs. 3A, B). In addition, we observed that in hippocampal cultures there was an association of p67phox with the synaptic marker synaptophysin (Fig. 3C). This association of p67phox and synaptophysin at resting state was modest; however, after stimulation with phorbol esters, the association of p67phox and synaptophysin increased, and the staining pattern of the cytoplasmic subunits alone was more punctate (Fig. 4). This close association between p67phox and synaptophysin was easily detected because the treatment of hippocampal cultures with PMA also reflected an increase in the integral membrane protein synaptophysin that was used as synaptic marker (Chapell and Rosenberg, 1996). These observations suggest that PMA induces translocation of the cytoplasmic NADPH oxidase subunits to the membrane near synaptic sites.
In phagocytes, the obligatory step for the activation of NADPH oxidase is the translocation of the cytosolic subunits (p40phox, p47phox, p67phox and Rac) to the plasma membrane, where they associate with membrane components, resulting in activation of the enzyme (Clark, 1990; DeLeo and Quinn, 1996; Quinn and Gauss, 2004). In the mouse hippocampus, this also appears to occur because stimulation of hippocampal slices with PMA also increased the translocation of the cytoplasmic subunits to the membrane (Fig. 5). Stimulation of hippocampal slices with PMA also resulted in the production of superoxide, which was blocked by two inhibitors of NADPH oxidase (Fig. 6). Taken together, these observations are consistent with a functional, membrane assembled NADPH oxidase being present in the hippocampus. Treatment of hippocampal slices with DPI alone also decreased basal levels of superoxide. It should be noted that DPI also is able to reduce mitochondrial superoxide production (Li and Trush, 1998), which recently was shown to increase the activity of several protein kinases in hippocampal neurons (Hongpaisan et al., 2004). However, PMA-induced superoxide formation also was reduced by AEBSF, an NADPH oxidase inhibitor that is functionally and structurally distinct from DPI (Diatchuk et al., 1997; Hancock and Jones, 1987). Thus, taken together, our findings are consistent with a PKC-dependent activation of NADPH oxidase in the hippocampal neurons after treatment with PMA. It is well known that incubation of hippocampal slices with phorbol esters increases synaptic transmission (Malenka et al., 1986, 1987; Parfitt and Madison, 1993). Therefore, our findings also suggest the possibility that production of superoxide via NADPH oxidase contributes to phorbol ester-induced increases in synaptic transmission in hippocampal slices. This possibility remains to be determined.
The expression of NADPH oxidase proteins in neurons suggests that enzymatic production of superoxide by NADPH oxidase plays a role in normal neuronal function. Consistent with this idea, NGF-induced neuronal differentiation has been shown to be dependent on ROS production via a NADPH oxidase-like protein (Suzukawa et al., 2000). In addition, we have postulated the NADPH oxidase to be a source of superoxide that is required for hippocampal long-term potentiation (LTP) and hippocampus-dependent memory (Knapp and Klann, 2002; Thiels et al., 2000). Our observations indicate that a functional NADPH oxidase is present in the hippocampus and is consistent with the notion that NADPH oxidase might be involved in hippocampal LTP and hippocampus-dependent memory. Interestingly, patients with chronic granulomatous disease (CGD), which is characterized by a deficient phagocytic NADPH oxidase (Rosenzweig and Holland, 2004), have been reported to suffer from cognitive deficits (Pao et al., 2004). It will be of great interest to determine whether p47phox-deficient mice that model CGD (Jackson et al., 1995) have LTP and/or hippocampus-dependent memory deficits.
Herein, we utilized the NBT assay to detect NADPH oxidase-dependent production of intracellular superoxide in hippocampal slices (Patterson et al., 1999). However, NADPH oxidase also has been shown to produce extracellular superoxide in the hippocampus (Patel et al., 2005). Previous studies have shown that cell permeable scavengers of superoxide block LTP (Klann, 1998) and that cell impermeable scavengers of superoxide attenuate LTP (Klann et al., 1998). Thus, it will be of interest to determine whether superoxide produced by NADPH oxidase functions as an intracellular messenger and/or a transcellular messenger molecule during hippocampal LTP.
NADPH oxidase also has been shown to play a role in pathophysiological processes such as ischemic stroke (Walder et al., 1997) and the MPTP model of Parkinson’s disease in the mouse (Wu et al., 2002). In addition, NADPH oxidase-dependent production of superoxide has been speculated to be involved in Alzheimer’s disease (Della Bianca et al., 1999; Hashimoto et al., 2002; Shimohama et al., 2000). The presence of a functional NADPH oxidase in the brain, including the hippocampus, cortex, and striatum, is consistent with the possibility that this enzyme plays a role in the neurodegeneration observed in stroke, Parkinson’s disease, and Alzheimer’s disease.
In this study, we have demonstrated the localization of NADPH oxidase proteins as well as the production of superoxide by an NADPH oxidase in the mouse hippocampus. Whether the NADPH oxidase proteins in the brain are identical to the well-characterized NADPH oxidase proteins in phagocytic cells or are components of a brain- and/or neuron-specific NADPH oxidase remains to be determined. There are several reports in which NADPH oxidase homologs have been characterized in diverse tissues such as the colon (Banfi et al., 2000; Suh et al., 1999), kidney (Geiszt et al., 2000), and thyroid (De Deken et al., 2000). Nevertheless, the demonstration of a functional NADPH oxidase in the mouse brain suggests that this enzyme is a possible source of superoxide that may contribute to both physiological processes and pathophysiological conditions in the nervous system.
Experimental methods
Brain homogenates and subcellular fractionations
Animals were handled and sacrificed in compliance with institutional and national regulations and policies. The protocol was approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Male C57BL6 mice (6–8 weeks old) were sacrificed by cervical dislocation under gentle anesthesia. The brain was removed and cortex, cerebellum, hippocampus, and brain stem were dissected and homogenized in buffer (10 mM HEPES, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 1 μM microcystin-LR, and 200 nM calyculin A). The homogenates were centrifuged at 1500 × g for 10 min at 4°C, followed by a second centrifugation of the supernatant at 20 psi for 45 min using an air-driven ultracentrifuge (Beckman Coulter, Palo Alto, CA), and separated into membrane and cytoplasmic fractions.
Hippocampal slice preparation and treatment with phorbol esters
Hippocampi from mice were removed and 400 μm slices were prepared using a vibratome. Slices were perfused for 1 h with a standard saline solution (5 mM glucose, 110 mM sucrose, 60 mM NaCl, 28 mM NaHCO3, 3 mM KCl, 1.25 mM NaH2PO4, 7 mM MgCl2, 0.5 mM CaCl2, and 0.6 mM ascorbate) and equilibrated with 95% O2/5% CO2. For pharmacological experiments, slices were transferred to individual chambers and maintained in artificial cerebrospinal fluid (ACSF, 25 mM glucose, 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1 mM MgCl2, and 2 mM CaCl2) for 1 h at 32°C and then exposed to 1 μM phorbol-12-myristate-13-acetate (PMA, Sigma, St. Louis, MO) for 5 min or to different compounds of interest for the indicated times. Slices then were homogenized, centrifuged, and separated into membrane and cytoplasmic fractions as described above.
Synaptoneurosome and postsynaptic density preparations
Synaptoneurosome and postsynaptic densities (PSDs) were isolated as described previously (Carlin et al., 1980; Heynen et al., 2000) with some modifications. Two sets of four whole brains or ten whole hippocampi from 6- to 12-week-old C57BL/6 male mice were homogenized in synaptoneurosome buffer (10 mM HEPES, 1 mM EDTA, 2 mM EGTA, 0.5 mM DTT, 10 μg/ml leupeptin, and 50 μg/ml soybean trypsin inhibitor) at 4°C using a tissue grinder. The brain material obtained was diluted to a 5% solution (w/v) in synaptoneurosome buffer followed by sonication. The samples then were filtered through a 100-μm nylon filter, then through a 30-μm nylon filter, and finally through a hydrophilic 5 μm filter (Millipore Co., Bedford, MA). The filtrates from each set of samples were pooled in a 50-ml polycarbonate open top tube (Beckman Instruments, Palo Alto, CA) and centrifuged for 10 min at 10,000 × g in a JA-14 rotor (Beckman Instruments) at 4°C. The resulting pellet containing synaptoneurosomes was thoroughly resuspended in solution B (1:6 w/v, 0.32 M sucrose, and 1 mM NaHCO3). A portion of these synaptoneurosomes was saved and analyzed via Western blots. The remaining sample was further resuspended in an equal volume of 1% Triton X-100 in 32 mM sucrose–12 mM Tris–HCl (pH 8.1). This solution was stirred for 15 min in an open polycarbonate tube (Beckman Instruments) and then centrifuged for 20 min at 33,000 × g in a 60 Ti Rotor (Beckman Instruments) at 4°C. The resulting pellet was suspended in 0.5 ml of solution B and briefly sonicated. Thereafter, the resuspended pellet was layered onto a sucrose gradient in a 4-ml polycarbonate open top tube containing 1.3 ml of 2 M sucrose, 1 ml of 1.5 M sucrose–1 mM NaHCO3 and 1 ml of 1 M sucrose–1 mM NaHCO3 and centrifuged for 2 to 4 h at 200,000 × g in a 70.1 Ti rotor (Beckman Instruments) at 4°C. The PSD containing band between the 2 M sucrose and 1.5 M sucrose–1 mM NaHCO3 layers was carefully removed. This band was extracted, transferred to a 4-ml polycarbonate tube, and diluted with 600 μl of solution B and 600 μl of 1% Triton X-100–150 mM KCl. The sample then was centrifuged at 200,000 × g for 12 min at 4°C. The resulting pellet was suspended in solution B and sonicated. A Bradford assay (Bradford, 1976) was performed to calculate the PSD protein yield.
Western blot analysis
Equivalent amounts of protein from each brain region or from the different fractions (membrane, cytosol, synaptoneurosome, PSD) were resolved via electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels (10% SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes, and incubated in Tris-buffered saline with Tween 20 (TTBS, 50 mM Tris–HCl [pH 7.5–8.0], 150 mM NaCl, and 0.1% Tween 20) containing 5% non-fat milk for 1 h. A panel of monoclonal and polyclonal antibodies raised against human NADPH oxidase subunits (Burritt et al., 1995; DeLeo and Ulman, 1996; DeLeo and Quinn, 1996) were used to identify these subunits in the different regions of the mouse brain. Blots were incubated overnight with the corresponding primary antibody (gp91phox, p22phox, p47phox, p40phox, and p67phox at 1:500 dilution), Rac antibody (1:10,000 dilution; Upstate Biotechnology, Lake Placid, NY), actin (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), or Na+/K+ ATPase (1:1,000 dilution; Upstate Biotechnology) at 4°C, washed three times for 10 min in TTBS, followed by incubation with a horseradish peroxidase-conjugated goat anti-mouse/rabbit IgG (1:5,000 Promega, Madison, WI). Blots were visualized using enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, England). Densitometric analysis of all samples was performed using Scion image software (Scion, Frederick, MD).
Superoxide determination
Superoxide production was determined as described previously (Pick, 1986; Tan and Berridge, 2000) with some modifications. Six-to 8-week-old C57BL/6 male mice were sacrificed by cervical dislocation under gentle anesthesia. Hippocampi were removed and 300 μm slices were prepared using a vibratome. Slices were perfused for 1 h with the standard saline solution described previously and equilibrated with 95% O2/5% CO2. Thereafter, approximately seven slices were transferred to individual chambers and maintained in ACSF containing 20 μg/ml catalase (to prevent the formation of hydrogen peroxide) for 1 h at 32°C and exposed to 1 mg/ml nitroblue tetrazolium (NBT, Calbiochem, San Diego, CA) for 3 min followed by 7-min exposure to 2.4 μM PMA (Sigma, St. Louis, MO) or vehicle (control). Another set of slices were preincubated with either 10 μM diphenyleneiodomium (DPI, Calbiochem) or 10 μM 4-(2-aminoethyl) benzenesulfonyl fluoride (AEBSF, Sigma) for 10 min or 1 h, respectively, followed by a 10-min incubation with NBT or 3 min incubation with NBT followed by 7 min incubation with PMA. The reaction was stopped by placing the slices in 0.25 N HCl. After each treatment slices were transferred to tubes and a solution of DMSO and protease inhibitor cocktail (Sigma) was added before the samples were sonicated. Protein concentrations were calculated using a Bradford protein assay (Bradford, 1976). Absorbance at 550 nm was determined on a spectrophotometer.
Hippocampal CA1/CA3-dissociated cultures
Hippocampal cell cultures were prepared as previously described (Wu et al., 2001a,b). Briefly, hippocampal CA1–CA3 regions were dissected from C57BL6 mice 0 to 3 days old, dissociated by trypsin treatment followed by triturization with a siliconized Pasteur pipette, and then plated onto coverslips coated with matrigel. Culture media consisted of minimal essential media, 0.6% glucose, 0.1 g/L transferin, 0.25 g/L-insulin, 0.3 g/L-glutamine, 5–10% fetal calf serum, 2% B-27 supplement, and 2 μM cytosine-D-arabinofuranoside. Cultures were maintained at 37°C in 5% CO2 humidified incubator.
Immunocytochemistry
Four-week-old neuronal cultures were fixed in 4% paraformaldehyde and 4% sucrose overnight at 4°C and then washed three times in phosphate buffer saline solution (PBS). Cells then were permeabilized with 0.1% Triton X-100 in PBS for 15 min, followed by 30 min incubation at room temperature in blocking buffer (4% normal goat serum in PBS). Cells were incubated with antibodies to the NADPH oxidase proteins (gp91phox, p22phox, p47phox, and p67phox, 1:500 dilution), synaptophysin (1:500 dilution, Abcam Inc., Cambridge, MA) or synaptotagmin (1:500 dilution, Abcam Inc.) for 2 h at room temperature and then rinsed three times with PBS. Previous studies have characterized and established the specificity of the NADPH oxidase antibodies used in this study (Dvorakova et al., 1999; Pagano et al., 1997). Cells then were incubated with a secondary antibody (CY3 or FITC, 1:500 dilution, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 30 min, washed 3 times with PBS, mounted, and analyzed using a Zeiss LSM 510 META confocal microscope system (Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical analyses were performed using Student’s t tests for normally distributed variables or Bonferroni’s test for multiple comparisons, to determine the significant differences between control and treatment groups. A P value of less than 0.05 was considered statistically significant.
Acknowledgments
This research was supported by the National Institute of Health grants NS34007 (E.K.), T32-HL07676 (F.S.), and AR042426 (M.T.Q.).
References
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Babior BM, Lambeth JD, Nauseef WM. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- Batot G, Martel C, Capdeville N, Wientjes F, Morel F. Characterization of neutrophil NADPH oxidase activity reconstituted in a cell-free assay using specific monoclonal antibodies raised against cytochrome b588. Eur J Biochem. 1995;234:208–215. doi: 10.1111/j.1432-1033.1995.208_c.x. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2696. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Bolscher BG, Koenderman L, Tool AT, Stokman PM, Roos D. NADPH: O2 oxidoreductase of human eosinophils in the cell-free system. FEBS Lett. 1990;268:269–273. doi: 10.1016/0014-5793(90)81025-j. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brozna JP, Hauff NF, Phillips WA, Johnston RB., Jr Activation of the respiratory burst in macrophages. Phosphorylation specifically associated with Fc receptor-mediated stimulation. J Immunol. 1988;141:1642–1647. [PubMed] [Google Scholar]
- Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ. Topological mapping of neutrophil cytochrome b epitopes with phage-display libraries. J Biol Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker GA, Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986;6:714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapell R, Rosenberg P. In Vitro binding of synaptic vesicles to the synaptic plasma membrane: lack of effect of β-bungarotoxin. Toxicon. 1996;34:339–349. doi: 10.1016/0041-0101(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Clark RA. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990;161:1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Cross AR, Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1627:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- de Mendez I, Leto TL. Functional reconstitution of the phagocyte NADPH oxidase by transfection of its multiple components in a heterologous system. Blood. 1995;85:1104–1110. [PubMed] [Google Scholar]
- DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase:molecular interaction of oxidase proteins. J Leukocyte Biol. 1996;60:677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Ulman DV. Assembly of the human neutrophil NADPH oxidase involves binding of P67phox and flavocytochrome b to a common functional domain in p47phox. J Biol Chem. 1996;271:17013–17020. doi: 10.1074/jbc.271.29.17013. [DOI] [PubMed] [Google Scholar]
- Della Bianca V, Dusi S, Bianchini E, Dal Prà I, Rossi F. β-amyloid activates the O2− forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin V, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Dvorakova M, Höhler B, Richter E, Burritt JB, Kummer W. Rat sensory neurons contain cytochrome b558 large subunit immunoreactivity. NeuroReport. 1999;10:2615–2617. doi: 10.1097/00001756-199908200-00032. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukocyte Biol. 1999;65:335–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta. 1995;1231:215–219. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of Renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JJ, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Ito Y, Kita Y, Terracita K, Nishimoto I. Neurotoxic mechanisms by Alzheimer’s disease-linked N141I mutant presenilin 2. J Pharmacol Exp Ther. 2002;300:736–745. doi: 10.1124/jpet.300.3.736. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- Heyworth PG, Curnutte JT, Nauseef WM, Volpp BD, Pearson DW, Rosen H, Clark RA. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991;87:352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongpaisan J, Winters CA, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinases signaling in hippocampal neurons. J Neurosci. 2004;24:10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, O’Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocytic NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuro-Psychopharmacol Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Klann E, Roberson ED, Knapp LT, Sweatt JD. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J Biol Chem. 1998;273:4516–4522. doi: 10.1074/jbc.273.8.4516. [DOI] [PubMed] [Google Scholar]
- Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70:1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev, Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Li Y, Trush M. Diphenyleneiodomium and NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- Lu DJ, Furuya W, Grinstein S. Involvement of multiple kinases in neutrophil activation. Blood Cells. 1993;19:343–351. [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Ayoub GS, Nicoll RA. Phorbol esters enhance transmitter release in rat hippocampal slices. Brain Res. 1987;403:198–203. doi: 10.1016/0006-8993(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Mizuki K, Kadomatsu K, Hata K, Ito T, Fan QW, Kage Y, Fukumaki Y, Sakaki Y, Takeshige K, Sumimoto H. Functional modules and expression of mouse p40phox and p67phox, SH3-domain-containing proteins involved in the phagocyte NADPH oxidase complex. Eur Biochem. 1998;251:573–582. doi: 10.1046/j.1432-1327.1998.2510573.x. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao M, Wiggs EA, Anastacio MM, Hyun J, DeCarlo ES, Miller JT, Anderson VL, Malech HL, Gallin JI, Holland SM. Cognitive function in patients with chronic granulomatous disease: a preliminary report. Psychosomatics. 2004;45:23–234. doi: 10.1176/appi.psy.45.3.230. [DOI] [PubMed] [Google Scholar]
- Parfitt KD, Madison DV. Phorbol esters enhance synaptic transmission by a presynaptic, calcium-dependent mechanism in rat hippocampus. J Physiol. 1993;471:245–268. doi: 10.1113/jphysiol.1993.sp019900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Ma M, Ruedi JM, Smith RM, Babior BM. The cytosolic components of the respiratory burst oxidase exist as a M(r) approximately 240,000 complex that acquires a membrane-binding site during activation of the oxidase in a cell-free system. J Biol Chem. 1992;267:17327–17332. [PubMed] [Google Scholar]
- Patel M, Li QY, Chang LY, Crapo J, Liang LP. Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J Neurochem. 2005;92:123–131. doi: 10.1111/j.1471-4159.2004.02838.x. [DOI] [PubMed] [Google Scholar]
- Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Gauss KA. Structure and regulation of the neutropil respiratory burst oxidase: comparison with non-phagocyte oxidases. J Leukocyte Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113:620–626. doi: 10.1016/j.jaci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Sadikot RT, Zeng H, Yull FE, Li B, Cheng DS, Kernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, Blackwell TS, Christman JW. p47phox deficiency impairs NF-κB activation and host defense in Pseudomonas pneumonia. J Immunol. 2004;172:1801–1808. doi: 10.4049/jimmunol.172.3.1801. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Shatwell KP, Segal AW. Molecule in focus. NADPH oxidase Int J Biochem Cell Biol. 1996;28:1191–1195. doi: 10.1016/s1357-2725(96)00084-2. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth J. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Wientjes FB, Hsuan JJ, Totty NF, Segal AW. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. 1993;296:557–561. doi: 10.1042/bj2960557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci. 2001a;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001b;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


