SUMMARY
Background
A 53-year-old female presented with a 10-year history of paroxysmal atrial fibrillation (AF), precipitated by activity and refractory to medical therapy. In the absence of traditional risk factors for disease, a genetic defect in electrical homeostasis underlying stress-induced AF was explored.
Investigations
Echocardiography, cardiac perfusion stress imaging, invasive electrophysiology with isoproterenol provocation, genomic DNA sequencing of KATP channel genes, exclusion of mutation in 2,000 individuals free of AF, reconstitution of channel defect with molecular phenotyping, and verification of pathogenic link in targeted knockout.
Diagnosis
KATP channelopathy caused by missense mutation (Thr1547Ile) of the ABCC9 gene conferring predisposition to adrenergic AF originating from the vein of Marshall.
Management
Disruption of arrhythmogenic gene-environment substrate at the vein of Marshall by radiofrequency ablation.
Keywords: ABCC9, arrhythmia, ATP-sensitive K+ channel, atrial fibrillation, genetics
This article offers the opportunity to earn one Category 1 credit toward the AMA Physician’s Recognition Award.
THE CASE
A 53-year-old white female presented with a 10-year history of daily paroxysms of atrial fibrillation (AF) that peaked with morning activity. At the time of presentation the paroxysms were increasing in frequency and associated with near syncope. The patient had been refractory to antiarrhythmic medical treatment with flecainide and propafenone, and intolerant to sotalol therapy because of symptomatic bradycardia while in sinus rhythm. Risk factors for AF, such as hypertension, hyperthyroidism, and family history, were absent. Myocardial, valvular and coronary artery disease were excluded by echocardiography and cardiac perfusion stress imaging. Ventricular wall thickness and atrial volume were normal.
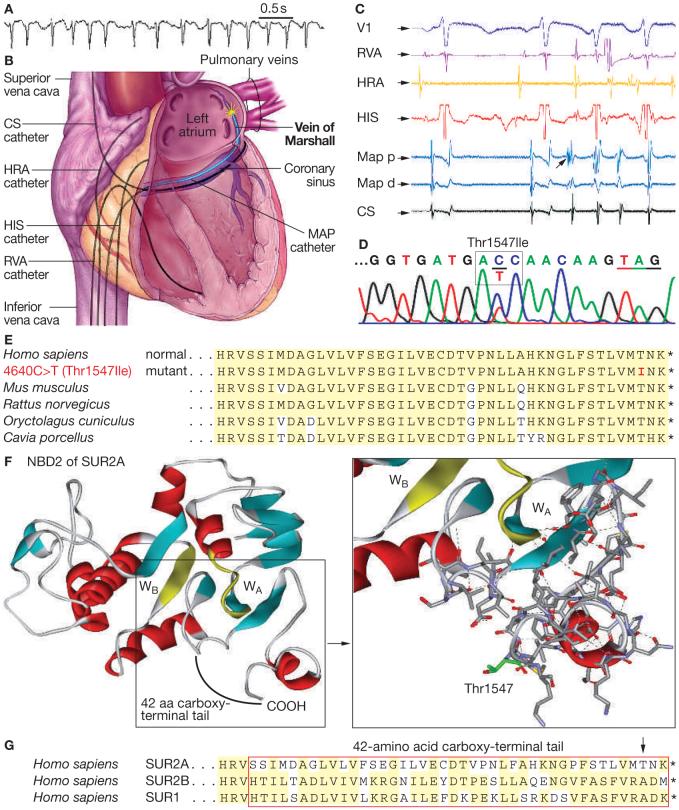
In this patient with early-onset AF and an overtly normal heart, adrenergic stress as a possible trigger was investigated by invasive electrophysiology, which documented recurrent atrial arrhythmia under sympathomimetic challenge with intravenous infusion of 2 μg/min isoproterenol (Figure 1A). A roving mapping catheter and differential pacing pinpointed a focal source of rapidly firing electrical activity within the vein of Marshall, which initiated AF under adrenergic provocation (Figure 1B,C).
Figure 1.
Mutation in ABCC9, which encodes the SUR2A KATP channel subunit, in a patient with AF originating from the vein of Marshall. (A) AF with variable ventricular rate response was provoked by isoproterenol infusion (2 μg/min). (B,C) Intracardiac mapping localized the site of earliest activation of premature AF-initiating beats to the vein of Marshall (arrow, C). (D) Sequencing of the patient’s DNA identified a heterozygous missense mutation (4640C>T) causing substitution of the 1547 threonine residue for isoleucine (Thr1547Ile) in exon 38 of ABCC9. Exon 38 encodes the carboxy-terminus of SUR2A. (E) Alignment of the human SUR2A carboxy-terminal domain with orthologs from other species demonstrated the conserved Thr1547 and surrounding region. (F) Model of SUR2A NBD2 with carboxy-terminal tail. Red denotes α-helix, blue-green β-strand, and yellow WA or WB (left panel). Thr1547, found to be substituted in the patient, mapped to the SUR2A tail region adjacent to Walker motifs. Dashed lines indicate hydrogen bonds that stabilize Walker A and the associated carboxy-terminus. Red denotes oxygen atoms, royal blue denotes nitrogen, and mustard denotes sulfur (right panel). (G) Alignment of human SUR isoforms indicated that the carboxy-terminal tail, including Thr1547 (arrow), is unique to cardiac SUR2A. Abbreviations: aa, amino acid; AF, atrial fibrillation; CS, coronary sinus; HIS, His bundle; HRA, high right atrial; Map, mapping; Map d, distal mapping catheter; Map p, proximal mapping catheter; NBD, nucleotide-binding domain; RVA, right ventricular apex; V1, surface electrocardiogram; WA, Walker A motif; WB, Walker B motif.
The patient underwent genetic evaluation, targeting cardiac ion channels1 including the ATP-sensitive potassium (KATP) channel. While not previously implicated in the pathogenesis of AF, the KATP channel was selected on the basis of its function in maintaining electrical stability under stress, including adrenergic challenge.2,3 Sequencing of KATP channel genes,4 using genomic DNA extracted from the patient’s peripheral white blood cells, demonstrated a missense mutation in ATP-binding cassette, sub-family C(CFTR/MRP), member 9 (ABCC9)—the gene encoding the regulatory SUR2A channel subunit. Identified in exon 38, specific for the cardiac splice variant of SUR2A, this heterozygous 4640C>T transition caused substitution of the threonine residue at amino acid position 1547 with isoleucine (Figure 1D). The patient’s first-degree relatives did not consent to clinical or genetic testing, precluding segregation analysis. To exclude the possibility that the Thr1547Ile substitution was a polymorphism in the general population, 4,000 chromosomes in 2,000 unrelated, healthy and predominantly white individuals were tested by heteroduplex analysis.4 All samples scanned were negative for the 4640C>T defect in exon 38, implicating the genetic anomaly as a pathogenic mutation.
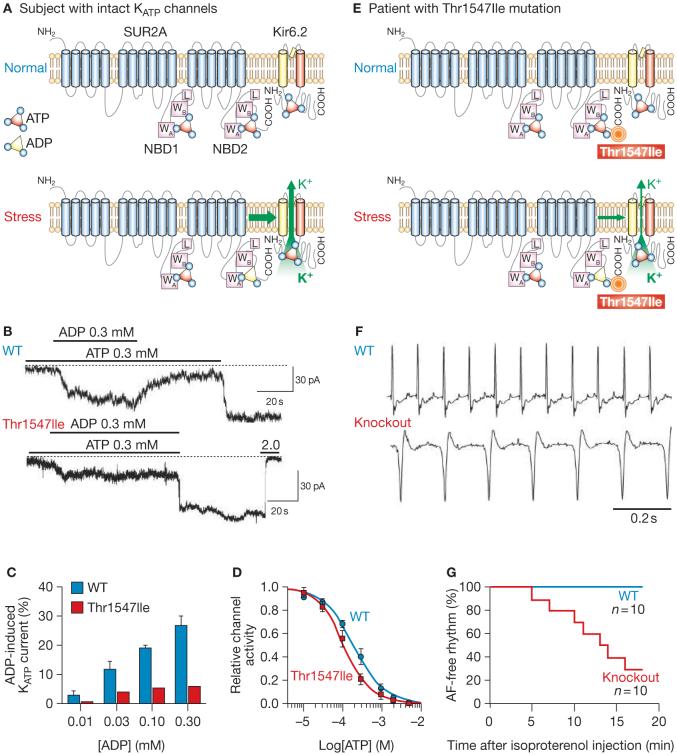
To determine the consequences of the patient’s Thr1547Ile mutation on channel structure, the regulatory SUR2A subunit of the KATP channel was compared with its orthologs and isoforms. Protein alignments revealed that the missense substitution altered the amino acid sequence of the evolutionarily conserved carboxy-terminal tail (Figure 1E). Homology modeling4 mapped the defect adjacent to the signature Walker motifs of the nucleotide binding domain, required for coordination of adenine nucleotides in the nucleotide binding pocket (Figure 1F).5 The carboxy-terminal tail, including the Thr1547 residue, is structurally and functionally unique, distinguishing the SUR2A isoform from non-cardiac SUR proteins (Figure 1G).5 Removal of the polar threonine (Thr1547) and replacement with the larger aliphatic and highly hydrophobic isoleucine, as would occur in this patient, predicted compromised nucleotide-dependent KATP channel gating (Figure 2A).
Figure 2.
The ABCC9 mutation disrupts KATP channel function, with the disease phenotype verified in an adrenergically stressed gene knockout model. (A) Cardiac KATP channels comprise the ABCC9-encoded SUR2A subunit (containing NBD1 and NBD2, each with WA, WB motifs and linker region), and the KCNJ11-encoded Kir6.2 pore. Intracellular ATP normally keeps KATP channels closed. Stress-induced build-up of MgADP at NBD2 of SUR2A antagonizes ATP-induced channel inhibition, promoting K+ efflux. (B) Compared with the WT subunit, mutant SUR2A (Thr1547Ile) coexpressed with Kir6.2 demonstrated aberrant KATP channel function, characterized by defective response to ADP in patch-clamp recordings. (C) Reduced K+ current response to escalating ADP concentrations in mutant (Thr1547Ile) versus WT KATP channels. (D) ATP-induced channel inhibition was retained in mutant channels. Channel inhibition was expressed relative to activity recorded in the absence of ATP at -60 mV, in inside-out patches. Curves represent Hill equation fits of data. (E) The Thr1547Ile mutation did not prevent ATP-induced KATP channel closure, but compromised ADP-dependent channel opening. (F) During telemetry in the conscious state, WT mice maintained sinus rhythm following isoproterenol (10 mg/kg) injection, whereas Kir6.2-/- knockout mice lacking functional KATP channels demonstrated—with sympathomimetic challenge—electrical vulnerability manifested by AF. (G) KATP channel deficit led to AF in 70% of knockout mice within 20 min of adrenergically medicated stress. Sinus rhythm was maintained in all WT mice with intact KATP channels. Abbreviations: AF, atrial fibrillation; L, linker region; NBD, nucleotide-binding domain; pA, picoAmps; WA, Walker A motif; WB, Walker B motif; WT, wild type.
To replicate the disease mutation and assess the effects of altered KATP channel structure on function, recombinant constructs of channel genes were expressed in cultured cells.4 Patch-clamp recording demonstrated that the Thr1547Ile substitution compromised adenine nucleotide-dependent induction of KATP channel current (Figure 2B). Mutant Thr1547Ile SUR2A, coexpressed with the KCNJII-encoded Kir6.2 pore, generated an aberrant channel that retained ATP-induced inhibition of potassium current, but demonstrated a blunted response to ADP (Figure 2B-D). A deficit in nucleotide gating, resulting from the Thr1547Ile mutation, would compromise the homeostatic role of the KATP channel required for proper readout of cellular distress and maintenance of electrical stability (Figure 2E). The pathogenic link between channel malfunction and adrenergic AF was verified, at the whole organism level, in a murine knockout model deprived of operational KATP channels.3 Compared with the normal atrium, resistant to arrhythmia under adrenergic provocation, vulnerability to AF was recapitulated in the setting of a KATP channel deficit (Figure 2F,G). Thus a lack of intact KATP channels, either because of a naturally occurring mutation affecting channel regulation or targeted disruption of the channel complex, is a substrate for atrial electrical instability under stress, and a previously unrecognized molecular risk factor for adrenergic AF.
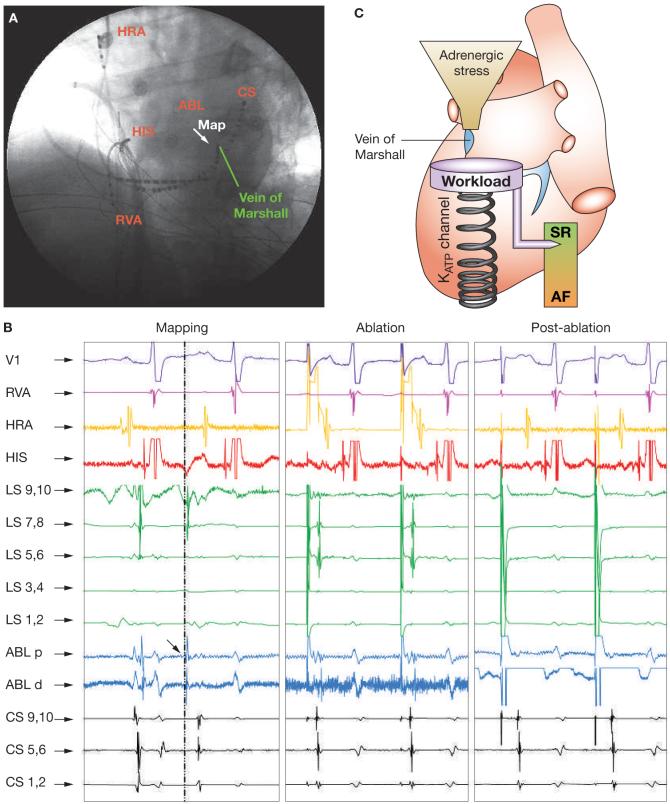
Failure of medical therapy, with refractoriness or intolerance to antiarrhythmic medication, was an indication for ablative intervention to restore sinus rhythm in the patient. Following mapping of the earliest activation sites for AF within the left atrium, radiofrequency energy was delivered to achieve electrical isolation of the initiating source (Figure 3A,B). Following ablation of the right superior pulmonary vein orifice, ectopy-initiating AF persisted, and was eliminated only after electrical isolation of the vein of Marshall (Figure 3B). In fact, once the vein of Marshall had been isolated, AF could no longer be provoked by programmed electrical stimulation and burst pacing with or without isoproterenol infusion (Figure 3B). Following ablation, the patient has remained in sinus rhythm and symptom-free over a 2.5 year follow-up period.
Figure 3.
Ablation of the vein of Marshall eliminated adrenergic AF in a patient with mutated KATP channels. (A) Fluoroscopic view of mapping catheter within the vein of Marshall at the site of the earliest atrial activation during isoproterenol-induced ectopy initiating AF. (B) Multilead intracardiac tracings before, during, and after radiofrequency ablation. Mapping: spontaneous atrial ectopy was first recorded (dotted and dashed line) in the ablation catheter, overlaying the vein of Marshall, where an abnormal potential (arrow) preceded atrial activation. Ablation: radiofrequency ablation eliminated the vein of Marshall potential and atrial ectopy. Postablation: despite isoproterenol infusion (2 μg/min), the vein of Marshall potential or ectopy could no longer be induced and the patient remained in sinus rhythm. (C) Adrenergic challenge provoked ectopy from the vein of Marshall, triggering transition from sinus rhythm to AF in the susceptible patient with inadequate adaptation to imposed stress load. As stress-responsive KATP channels preserve electrical homeostasis, channel malfunction resulting from gene mutation creates a risk-conferring substrate, predisposing to arrhythmia. Ablation of the arrhythmia-triggering source disrupted the arrhythmogenic gene-environment interaction. Abbreviations: ABL d, distal electrodes of ablation catheter; ABL p, proximal electrodes of ablation catheter; AF, atrial fibrillation; CS, coronary sinus catheter (distal, proximal); HIS, His bundle recording catheter; HRA, high right atrial catheter; LS, left superior pulmonary vein catheter (distal, proximal); Map, mapping catheter; RVA, right ventricular apex catheter; SR, sinus rhythm; V1, surface electrocardiogram.
DISCUSSION OF DIAGNOSIS
AF is an electrical disorder characterized by chaotic atrial activation, defined on the electrocardiogram as replacement of sinus P waves by rapid oscillations or fibrillatory waves associated with an irregular ventricular response. A growing epidemic in the aging population with structural heart disease, AF also presents as an earlier-onset, apparently idiopathic, condition in a subset of patients6 and is increasingly recognized as a heritable disorder.7 The paradigm of a genetic basis for AF is exemplified by reports of familial disease attributed to gain-of-function8 or loss-of-function1 mutations in ion channel genes predicted to accelerate or slow repolarization. In these cases, channel malfunction creates an arrhythmogenic milieu of re-entry or triggered activity caused by reduced electrical refractoriness or after-depolarization, respectively.1,8 AF is thus emerging as an inherited channelopathy,1,7,8 although the molecular basis for this arrhythmia in the majority of patients remains undefined.
In this case of early-onset lone AF, the medical history and the diagnostic electrophysiological study established a lack of traditional risk factors, but a vulnerability to adrenergic stress—a known trigger of arrhythmogenesis.9 Electroanatomical interrogation localized the initiating arrhythmogenic trigger to a veno-atrial interface. Premature atrial ectopy that increased in the presence of isoproterenol was mapped to the vein of Marshall. This vestige of the embryonic left superior vena cava, rich in sympathetic innervation, is a recognized catecholamine-sensitive focus of atrial ectopy.10 Although this potentially arrhythmogenic veno-atrial interface is present in the population at large, it does not trigger arrhythmia in the majority of individuals.11 In certain patients, vulnerability to adrenergic AF in the absence of structural heart disease, as in this case, implicates an inherent defect in electrical stability. Why certain patients develop vein-of-Marshall-triggered AF while others do not, despite comparable environmental stress exposure, might ultimately depend on their genetic make-up.
While family history did not specifically point to a genetic defect in this patient, the apparent lack of AF among first-degree relatives did not preclude molecular genetic investigation, as a de novo or incompletely penetrant mutation could account for a negative family history. Selection of KATP channels for mutation screening was based on the unique property of these metabolism-sensing channels to operate as molecular stress-responsive rheostats 2. The KATP channel is formed by the regulatory SUR2A subunit, which harbors nucleotide binding domains, and Kir6.2, the conduit for potassium ions.12 Under increased cardiac workload, build-up of intracellular ADP at SUR2A translates into channel opening and action potential shortening, limiting calcium overload induced by adrenergic stimulation.2,3 Gene expression and electrophysiological studies in patients with AF have demonstrated altered atrial ion channel mRNA transcription and post-translational activity, including downregulation of the KATP channel pore and associated current.13,14 Moreover, genetic deficits in KATP channels have recently been linked to myopathic heart disease and electrical instability.4 In fact, deficient repolarization reserve under KATP channel malfunction predisposes to early after-depolarization and the induction of triggered activity under sympathetic stress.15 Upon identification of an ABCC9 sequence variant in the patient’s genomic DNA, a comprehensive algorithm was implemented. Initial genetic screening for the variant in a control population was followed by in silico protein modeling, verification of mutant channel malfunction by patch-clamp electrophysiology, and linkage to disease susceptibility through gene targeting. This individualized approach enabled the molecular diagnosis of KATP channel dysfunction to be established.
DIFFERENTIAL DIAGNOSIS
Recurrent palpitations in a young person with a structurally normal heart could be attributable to supraventricular arrhythmia involving accessory pathways, atrioventricular node re-entry tachycardia, or atrial tachycardia, all of which were excluded in this patient by surface electrocardiography and invasive electrophysiology. Catecholamine-sensitive early-onset arrhythmias can also originate in the ventricle, as is the case with right ventricular outflow tract tachycardia, catecholaminergic polymorphic ventricular tachycardia, and arrhythmogenic right ventricular myopathy. These conditions were excluded by cardiac imaging, electrocardiography and invasive electrophysiology, which demonstrated that electrical activation originated in the vein of Marshall. A subset of patients with an apparently idiopathic form of AF could have an underlying cardiac channelopathy. Lone AF has been linked to mutations in genes encoding voltage-gated or inwardly-rectifying potassium channels,1,7,8 yet in this case genetic and functional tests uncovered a defect in the ATP/ADP-gated SUR2A subunit, indicating a KATP channelopathy.
TREATMENT AND MANAGEMENT
AF in this patient was refractory to medical therapy with sodium and potassium channel inhibitors, and anti-adrenergic therapy was poorly tolerated owing to adverse effects. Currently, KATP channel opening drugs are not approved for the treatment of rhythm disorders. The apparently curative outcome following ablation of the vein of Marshall in this patient would indicate that vulnerability to arrhythmia was caused by a combination of localized susceptibility to adrenergic stress, and an inability of mutant KATP channels to safeguard against catecholamine-induced ectopy (Figure 3C). Radiofrequency ablation is indicated for symptom control in highly symptomatic patients with AF refractory to medical therapy, yet no randomized clinical trial comparing pharmacotherapy to ablation has yet been conducted. AF did not recur despite persistence of genetically defective KATP channels. This result indicates that elimination of the arrhythmogenic source—prone to catecholamine-induced electrical instability10— disrupted the arrhythmogenic gene-environment interaction, and was sufficient to overcome the inherent intolerance at the vein of Marshall to ongoing adrenergic stress (Figure 3C). This outcome is in line with successful treatment of ventricular fibrillation in genetic arrhythmia syndromes by ablation of triggers from the Purkinje conducting system arborization or the right ventricular outflow tract.16 Notwithstanding, defective KATP channels remain throughout the patient’s atrial myocardium despite focal ablation, and adrenergic AF might recur with advancing age and cumulative risk load despite electrical isolation of the vein of Marshall. While gene therapy and pharmaco-therapy are not currently applicable for targeted rescue of mutated channels, recurrence of AF in this KATP channelopathy could be responsive to reduction of upstream adrenergic burden (by β-adrenergic blockade or permanent pacing) or of downstream calcium overload (by calcium channel blockade).3
CONCLUSION
We identified a genetic defect in the homeostatic KATP channel of a patient with a structurally normal heart and a catecholamine-sensitive vein of Marshall trigger for AF, uncovering a molecular basis for impaired atrial stress responsiveness and susceptibility to early-onset disease. Discovery of the ABCC9 mutation represents the first known instance of a genetic defect in adrenergic, focal AF. This case thus defines KATP channel dysfunction as a substrate for atrial arrhythmia triggered by environmental stress (Figure 3C), and establishes a novel KATP channelopathy17 in human disease. Subsequent targeted screening for the Thr1547Ile SUR2A mutation in patients (n = 154) with diverse presentations of AF indicate that this specific genetic substitution is not common. Future research will be required to determine the overall mutation frequency within KATP channel genes and the spectrum of genotype-phenotype relationships. While the case underscores heritable channel dysfunction in lone AF, KATP channel deficit could play a broader role in the pathogenesis of electrical instability. Metabolic2 and mechanosensitive18 gating of KATP channels might indeed become compromised with structural heart disease and atrial dilation, precipitating suboptimal repolarization reserve and providing a substrate for the more common acquired form of AF.
Acknowledgments
The study was approved by the Mayo Clinic Institutional Review Board and Institutional Animal Care and Use Committee. This work was supported by the National Institutes of Health, Marriott Heart Disease Research Program, Marriott Foundation and Mayo Clinic. AE Alekseev is affiliated with the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, Puschino, Russia, and C Moreau is currently at the French National Center for Scientific Research, Molecular and Cellular Biophysics, Grenoble, France.
Footnotes
Competing interests
The authors declared they have no competing interests.
References
- 1.Olson TM, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 2.Alekseev AE, et al. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zingman LV, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bienengraeber M, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, et al. Intramolecular interaction of SUR2 subtypes for intracellular ADP-Induced differential control of KATP channels. Circ Res. 2002;90:554–561. doi: 10.1161/01.res.0000012666.42782.30. [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 7.Roberts R. Mechanisms of disease: genetic mechanisms of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2006;3:276–282. doi: 10.1038/ncpcardio0509. [DOI] [PubMed] [Google Scholar]
- 8.Chen YH, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 9.Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 10.Doshi RN, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 11.Haissaguerre M, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki N, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 13.Brundel BJ, et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 14.Balana B, et al. Decreased ATP-sensitive K+ current density during chronic human atrial fibrillation. J Mol Cell Cardiol. 2003;35:1399–1405. doi: 10.1016/s0022-2828(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu XK, et al. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 16.Haissaguerre M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 17.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]