Abstract
Background and Aims
Lymphocytes populate the livers of infants with biliary atresia, but it is unknown whether neonatal lymphocytes regulate pathogenesis of disease. Here, we investigate this question by examining the role of T lymphocytes in the destruction of extrahepatic bile ducts of neonatal mice using an experimental model of biliary atresia.
Methods
Inoculation of neonatal mice with rhesus rotavirus followed by multi-staining flow cytometry to quantify expression of Ifng by hepatic lymphocytes, and real-time PCR for mRNA expression of pro-inflammatory cytokines. This was followed by determining the consequences of antibody-mediated depletion of lymphocyte subtypes on the development of biliary obstruction, and co-culture and cell transfer experiments to investigate the effector role of lymphocyte subtypes on neonatal biliary disease.
Results
Rotavirus infection results in overexpression of Ifng by neonatal hepatic T cells. Among these cells, depletion of CD4+ cells did not change the course of inflammatory injury and obstruction of neonatal bile ducts. In contrast, loss of CD8+ cells remarkably suppressed duct injury, prevented luminal obstruction, and restored bile flow. Co-culture experiments showed that rotavirus-primed, but not naïve, CD8+ cells were cytotoxic to cholangiocytes. In adoptive transfer experiments, we found that primed CD8+ cells preferentially homed to extrahepatic bile ducts of neonatal mice and invaded their epithelial lining.
Conclusion
Primed neonatal CD8+ cells can activate a proinflammatory program, target diseased and healthy duct epithelium, and drive the phenotypic expression of biliary atresia, thus constituting a potential therapeutic target to halt disease progression.
Introduction
Exposure of newborns to the extra-uterine environment challenges the neonatal immune system to adapt to environmental antigens. In the immediate postnatal period, the cellular components of the immune system differ from adults both quantitatively and qualitatively, and display an adaptive response that is limited in magnitude and somewhat skewed to a Th2 phenotype.1, 2 These differences notwithstanding, when exposed to strong Th1-inducing agents, neonatal cytotoxic T lymphocytes can elicit competent cytotoxic responses.3-5 However, it is unclear whether neonatal lymphocytes can execute the entire biological program typical of organ-specific autoimmunity and cause neonatal disease. Here, we investigate this question by examining the role of neonatal T lymphocytes in the destruction of extrahepatic bile ducts in an experimental model of biliary atresia.
Biliary atresia is the most common form of chronic liver disease in children, and the leading indication for pediatric liver transplantation worldwide. The disease, which presents as pathologic jaundice during the neonatal period, is due to a selective injury to bile ducts, followed by an exuberant inflammatory and fibrosing response that obstructs the extrahepatic bile ducts, cessation of bile flow, and progression to cirrhosis.6 The causes of disease are largely undefined, but the exclusive onset of symptoms within 3 months of life and the coexistence of non-biliary congenital malformations have served as basis for the hypothesis that developmental factors play a key role in disease pathogenesis.6 To date, sequence analyses of genes that regulate biliary development have failed to identify disease-causing mutations.7 In contrast, a comprehensive survey of the hepatic transcriptome and tissue analyses uncovered molecular signatures and cellular phenotypes consistent with a prominent Th1 response in the early phases of neonatal disease.8, 9 A more direct cause-and-effect relationship emerged from mechanistic studies in which the constitutive loss of interferon-gamma (Ifng) prevented the inflammatory obstruction of bile ducts in a mouse model of biliary atresia induced by neonatal challenge with rhesus rotavirus (RRV).10 In this model, inoculation of RRV soon after birth results in an inflammatory obstruction of extrahepatic bile ducts within 7 days of life, with histological features similar to the disease in humans.11 Although the cellular basis of Ifng-driven obstruction of bile ducts and its relationship to autoimmunity have not yet been established, the liver and bile ducts activate a proinflammatory program and are infiltrated by committed Th1 lymphocytes.10 Further, a recent study suggested that autoreactive T lymphocytes recognize bile duct epithelial cells.12 Therefore, we used this experimental model to interrogate the effector role of developing lymphocytes in the targeted injury of bile ducts, and to explore the potential involvement of autoimmunity in the pathogenesis of experimental biliary atresia.
Materials and Methods
Mouse model and depletion of T lymphocyte subtypes
Within 24 hours of birth, Balb/c mice were injected with 1.5 × 106 fluorescence-forming units (ffu) of RRV or 0.9% NaCl (saline) solution intraperitoneally and housed with their mother in a specific pathogen-free vivarium in a room with a 12-hour dark-light cycle as described previously.10 Half of RRV- or saline-injected mice also received daily intraperitoneal injections of 80 μg of either rat anti-mouse CD4+ (GK1.5) or CD8+ (GK2.43) cell-depleting antibodies from days 2-5 of life, then again from 11-13 days according to a protocol previously shown to result in >99% loss of circulating CD4+ or CD8+ cells as shown by flow cytometry.13, 14 Groups of mice were sacrificed at 1-14 days, and livers and bile ducts were either snap frozen in liquid nitrogen, used for isolation of mononuclear cells, or paraffin-embedded. The number of mice in each experiment is presented in the Results, in tables or in figure legends. The Institutional Animal Care and Use Committee of the Cincinnati Children's Research Foundation approved all animal protocols.
Flow cytometric analysis
Mononuclear cells were isolated from peripheral blood or liver as described previously, following liver perfusion with saline through the portal vein.10 Cellular phenotyping was performed based on cell surface markers as described previously,10 using the following fluorochrome-conjugated antibodies: CD3 (17A2), CD4 (RM4-4 or RM4-5), CD8a (53-6.7), and Pan-NK (CD49b, DX5) from BD Biosciences (San Jose, CA, USA); isotype-matched immunoglobulin was used as negative controls. Additional intracellular staining to detect Ifng was performed using Cytofix/Cytoperm kit (GolgiPlug; Pharmingen, San Diego, CA) and eBioscience Intracellular Fixation and Permeabilization buffers (eBiosciences, San Diego, CA) according to the manufacturer's instruction, and with anti-Ifng antibody (XMG1.2). Flow cytometry was done with a FACSCalibur analyzer, capturing 100,000-150,000 events for each sample; results were analyzed with CellQuest software (BD Biosciences).
Cytotoxic assay
CD4+ or CD8+ T lymphocytes were purified from hepatic mononuclear cells at 7 days after saline- or RRV-injection using magnetic activated cell sorting (MACS) with negative selection and an AutoMACS machine according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA, USA). Enriched CD4+ or CD8+ cells were washed twice and immediately co-cultured in increasing ratios with a murine cholangiocyte cell line (mCL) on RPMI medium supplemented with 10% heat-inactivated fetal bovine serum.10, 15 Cholangiocytes served as target cells in cytolytic assays, were either naïve or RRV infected as described previously,10 were labeled with 51Cr, and were cultured with increasing concentrations of CD4+ or CD8+ cells for 5 hours.16, 17 The murine 67-NR breast cancer cell line was also used as target cells in cytolytic assays as controls because it lacks lineage identity to cholangiocytes or enterocytes, which are known to be targeted by rotavirus.18 Assays were done in triplicate wells, and a response was considered positive if specific lysis was at least twice the value of the negative control.
Adoptive transfer of T cell subsets
Single cell suspensions of hepatic CD4+ or CD8+ cells isolated from newborn mice 7 days after saline or RRV injection were transplanted into Balb/c-Rag2-/- newborn mice between 2-4 days of life; 8 × 105 cells in 30 μl saline solution were injected intraperitoneally. The absence of RRV in transplanted cells was demonstrated negative by fluorescence foci assay as described previously.10 Flow cytometric analysis of cell aliquots demonstrated >99% purity for CD4+ or CD8+ labeled cells. Mice were sacrificed 7 days after adoptive transfer, and homing of transplanted cells to the hepatobiliary system (extrahepatic bile ducts and liver) was determined with dual immunostaining in sections of paraffin-embedded tissues; lungs and kidneys were examined as control tissues.
Histopathology, immunostaining, and real-time PCR
Histopathological examination was performed in sections of microdissected extrahepatic bile duct/gallbladder after fixation and paraffin-embedding as described previously.10 For dual immunostaining, sections were incubated with rat anti-cytokeratin 8 (University of Iowa Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and rabbit anti-CD3 (Calbiochem, La Jolla, CA, USA) primary antibodies, followed by fluorochrome-labelled, species-specific antibodies and mounting medium containing 4′,6-diamidino-2-phenylindole. Specific signal was visualized with Nikon Eclipse E600 microscope (Nikon, Mellville, NY, USA), and labeled cells were counted and expressed as the total number of cells in the subepithelial (periductal) and intraepithelial regions of the entire extrahepatic bile duct. Real-time PCR was used to detect the level of mRNA encoding IL-2, IL12p40, TNFα, Cxcl9, 10 and 11 as described previously and using primers listed in Supplementary Table 1.
Statistical analysis
Values are expressed as mean ± standard deviation (S.D.), and statistical significance was determined by unpaired t test with a significance level set at P<0.05.
Results
Neonatal induction of Ifng by hepatic CD4+ and CD8+ cells in experimental atresia
The suitability of the RRV-induced model of bile duct injury to study postnatal development of the immune system is strongly supported by: 1) the narrow window of susceptibility to RRV challenge limited to the first few postnatal days, and 2) the stepwise progression from a well defined epithelial injury to ultimate luminal obstruction of bile ducts.10, 11 As seen in children with biliary atresia, livers of neonatal mice with experimental atresia undergo expansion of CD3+ cells and overexpress Ifng at the time of duct obstruction.8, 19 We previously reported that the loss of Ifng attenuates the tropism of lymphocytes to bile ducts and prevents the development of the biliary atresia phenotype.10 Therefore, we hypothesized that neonatal hepatic CD3+ cells that express Ifng induce the development of biliary atresia. To test this hypothesis, we first inoculated 1.5 × 106 ffu of RRV or saline (control) intraperitoneally into Balb/c mice in the first day of life and quantified the hepatic lymphocytes expressing Ifng by flow cytometry. The number of hepatic CD3+ cells increased in RRV-injected mice at 7 days (RRV = 3.1±1×106 versus saline = 0.5±0.02×106 cells, P<0.01) and 14 days of age (RRV = 7.3±2.2×106 and saline = 2±1×106 cells, P<0.02). We reported previously that hepatic CD3/CD4+ and CD3/CD8+ cell subtypes increase after RRV challenge.10 Here, we used dual flow cytometric analysis to also quantify NKT cells, and found that the population of NK cells co-stained with CD3 or CD4 is low in the first 7 days and increases after RRV challenge (Supplementary Fig. 1). Next, based on the well-recognized roles of CD4+ and CD8+ cells in the Ifng-rich adaptive immune response, we next quantified the number of Ifng-producing CD4+ and CD8+ cells following RRV challenge. In saline-injected controls, minimal or no Ifng was detected in CD4+ or CD8+ cells in the first 14 days of life; in contrast, the number of CD4+ cells expressing Ifng increased as early as day 3 after RRV challenge and peaked at the time of bile duct obstruction (day 7; Fig. 1A). A similar time course of increased Ifng expression was found in CD8+ cells (Fig. 1B). Because of previous reports suggesting that the administration of antigens to neonatal mice can elicit a Th1 response but may not support a tissue-specific inflammatory reaction,20-23 we searched for evidence of a direct effector link between CD3 cell subtypes and biliary injury.
Figure 1. Increased expression of Ifng by hepatic CD4+ and CD8+ cells after RRV challenge.
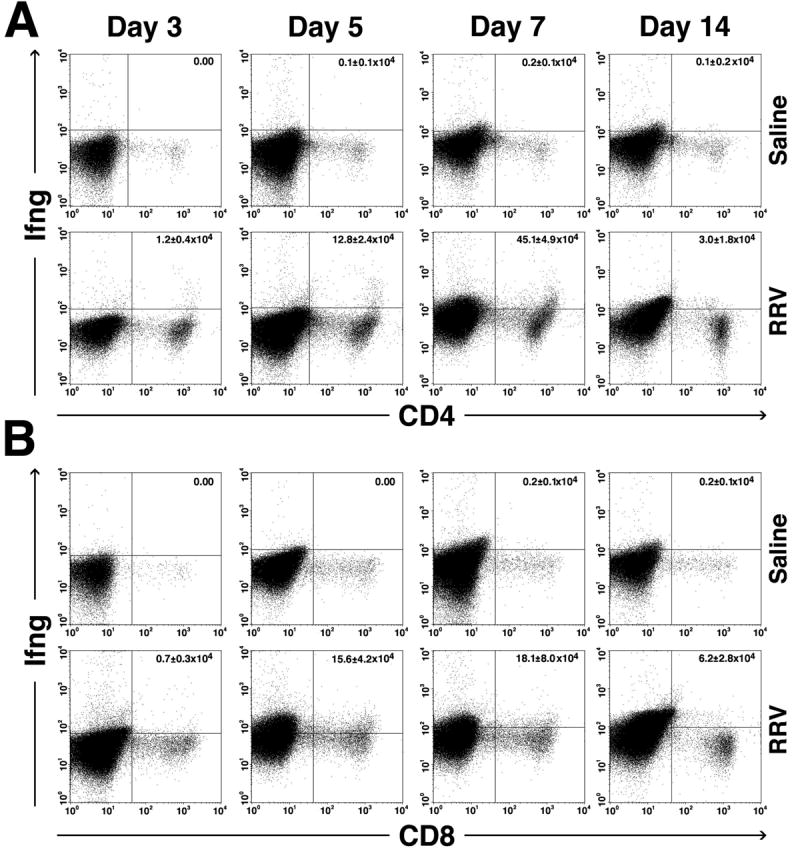
Dot plots of flow cytometric analyses after surface and cytoplasmic staining of hepatic mononuclear cells with anti-CD4 or CD8 and Ifng antibodies show an increase in the number of CD4+ and CD8+ cells expressing Ifng as early as 3 days after RRV challenge, with peaks at the time of obstruction of extrahepatic bile ducts (7 days). Minimal to no dual staining is present in cells from saline-injected (control) mice. The numbers in right upper quadrants represent the mean ± standard deviation for the total number of hepatic CD4+ or CD8+ cells expressing Ifng. Each group contains N=3 mice per time point; P<0.05 when the RRV group is compared to the saline group for each time point.
CD4-independent role of CD3/CD8+ cells in neonatal bile duct obstruction
To determine the impact of depletion of individual cell types in the development of biliary atresia, we first inoculated RRV or saline to newborn mice at day 1 of life, and subjected half of the mice in each group to daily injections of 80 μg of CD4-depleting monoclonal antibodies intraperitoneally.14 Antibody injections resulted in elimination of >99% of CD4 lymphocytes in peripheral blood (not shown); antibody injections also resulted in the prompt elimination of all CD4+ cells in the liver within 1 day after saline or RRV challenge, probably due to the low abundance of these cells in the liver during the immediate neonatal period (Supplementary Fig. 2). The injections were well tolerated as demonstrated by normal growth beyond 14 days in the saline-injected control group that also received CD4-depleting antibodies. In the group challenged with RRV, loss of CD4+ cells caused a minor delay in the onset of jaundice and acholic stools, but did not modify the final appearance of symptoms in all mice in a fashion similar to mice not injected with anti-CD4 antibodies (Fig. 2A), and did not impair the ability to clear RRV from the liver by 14 days (Supplementary Table 2). Histological analysis showed that the symptoms resulted from injury to the duct epithelium and invasion and obstruction of the lumen by inflammatory cells (Fig. 2B,C and Table 1), demonstrating that CD4+ cells are not required for the development of the biliary atresia phenotype.
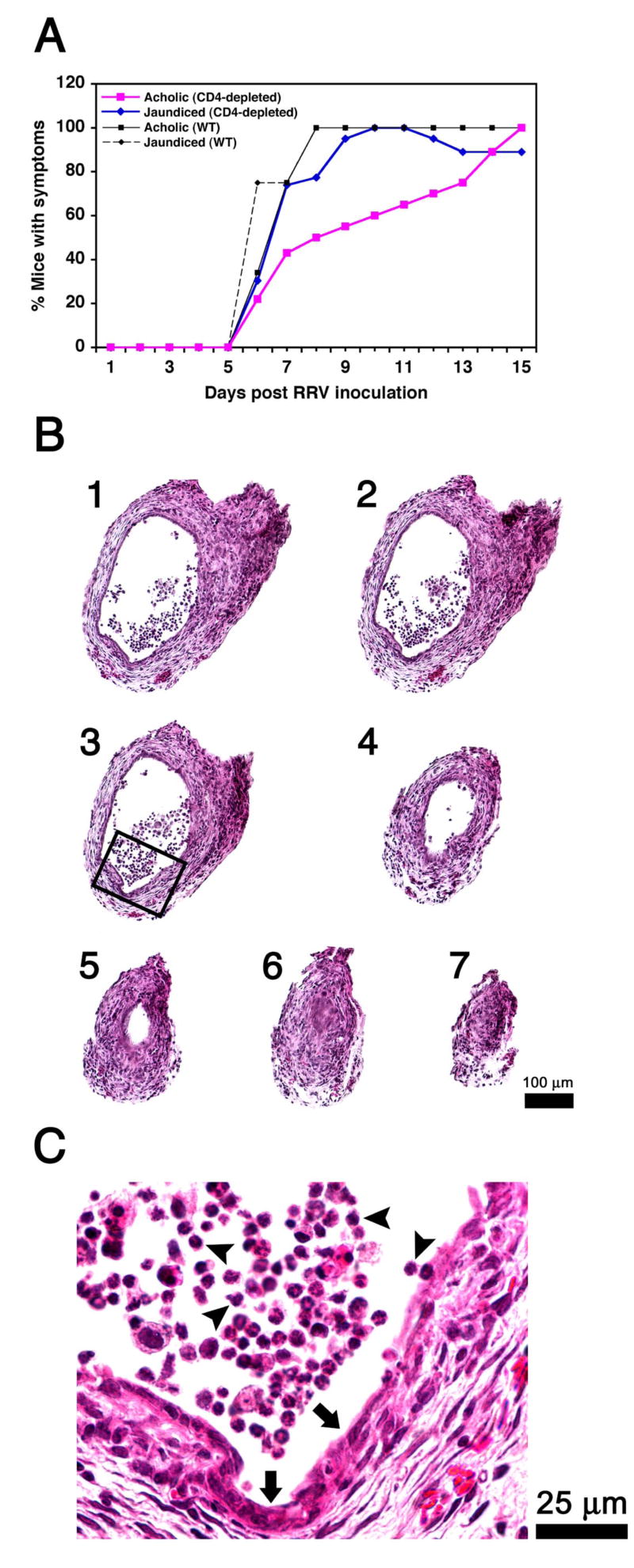
Figure 2. Loss of CD4+ cells does not modify the course of bile duct obstruction.
The onset of jaundice and acholic stools following RRV challenge in Balb/c mice was mildly delayed but the final appearance of symptoms in all mice was not altered by the antibody-mediated depletion of CD4+ cells (A). In B, successive cross-sectional views of bile ducts of CD4-depleted mice show infiltration of duct lumen by inflammatory cells (sections 1-4) and luminal obstruction (sections 5-7). The rectangle in section #3 is magnified in C, depicting epithelial injury and loss (arrows) and adjacent inflammatory cells (arrowheads). Hematoxylin/eosin stain; N=22 for WT mice infected with RRV; N=24 for CD4-depleted mice infected with RRV; sections are numbered from 1 to 7 to denote direction from the liver to the duodenum; WT = wild-type mice not injected with antibodies.
Table 1.
Percent of bile ducts with luminal obstruction as determined by examination of the entire length of extrahepatic bile ducts in sections stained with hematoxylin and eosin. Bile ducts were harvested from RRV-infected mice between days 12-14 post infection.
| Experimental Group | Number of mice investigated | % Obstructed bile duct |
|---|---|---|
| WT | 14 | 100 |
| CD4-depleted | 25 | 100 |
| CD8-depleted | 14 | 29 |
Depletion of CD8+ cells using a similar protocol with daily injection of 80 μg of anti-CD8 antibodies was also well tolerated, and eliminated >99% of circulating and hepatic CD8+ cells (results not shown).14 Interestingly, the time of onset of symptoms in CD8-depleted mice was similar to control mice challenged with RRV; however, these symptoms were short-lived, with gradual resolution of jaundice and acholic stools in most mice (Fig. 3A) despite the inability to clear virus from the liver (Supplementary Table 2). Histological analysis showed patent duct lumens maintaining biliary continuity between the liver and duodenum (Fig. 3B,C and Table 1). Although there was minimal or no evidence of epithelial injury, inflammatory cells were present in the bile duct wall (pericholangitis). The development of pericholangitis but absence of epithelial injury or obstruction pointed to a two-step biological setting in which pericholangitis develops independently of CD8+ cells, but epithelial injury and progression to duct obstruction require CD8+ cells. Further, the persistence of RRV in livers of mice without obstruction of bile ducts due to the loss of CD8+ cells suggests that the obstruction relates more directly to the inflammatory response, rather than to a direct viral effect.
Figure 3. Loss of CD8+ cells improves symptoms and prevents luminal obstruction of bile ducts.
RRV challenge of newborn mice induced the timely onset of jaundice and acholic stools in experimental and control groups. However, loss of CD8+ cells resulted in the resolution of symptoms in most mice by day 12 post infection (A). In B, successive cross-sectional views of bile ducts of CD8-depleted mice show infiltration of the subendothelial space by inflammatory cells (pericholangitis), but lack of intraluminal inflammation. The rectangle in section #1 is magnified in C, showing subepithelial inflammation (arrowheads) and intact epithelium (arrows). Hematoxylin/eosin stain; N=22 for WT mice infected with RRV; N=21 for CD8+ depleted mice infected with RRV; numbers 1 to 6 to denote direction from the liver to the duodenum; WT = wild-type mice not injected with antibodies.
Neonatal induction of inflammatory circuits despite the loss of CD4+ or CD8+ cells
The biliary phenotype in CD8-depleted mice was strikingly similar to the phenotype previously reported for Ifng-deficient mice.10 Therefore, we investigated whether impairment in Ifng expression by hepatic lymphocytes was one of the mechanisms of improved phenotype in CD8-depleted mice. To this end, we quantified the number of hepatic lymphocytes expressing Ifng in CD4- or CD8-depleted mice after saline or RRV challenge. The number of Ifng-producing T cell subsets increased 2.5-4.5-fold after RRV challenge in both CD4- and CD8-depleted mice (Fig. 4). To more broadly determine the induction of other proinflammatory signals, we used real-time PCR and found a similar pattern in the rise of hepatic mRNA expression for IL-2, IL12p40, Tnfα, and Cxcl9, 10 and 11 after RRV challenge in livers of CD4- and CD8- depleted mice, although both groups were generally lower than in non-depleted wild type mice inoculated with RRV (Supplementary Figure 3). These data imply that neonatal T lymphocytes and other liver cells can activate a broad inflammatory network in response to RRV independent of CD4+ or CD8+ cells. They also show that despite the previously described role of Ifng in regulating biliary tropism and obstruction by inflammatory cells, Ifng alone is not sufficient to produce duct obstruction; instead, it appears to require CD8+ cells to drive the inflammatory obstruction of the duct lumen.
Figure 4. Depletion of CD4+ or CD8+ cells does not modify expression of Ifng by lymphocytes in response to RRV.
Flow cytometric analysis depicting an increased number of Ifng-expressing CD4+ or CD8+ cells in livers of mice injected with RRV or saline in the first day of life. All mice were subjected to antibody depletion of either CD4+ (red) or CD8+ (blue) cells; N=3 mice per group/time point; *P<0.05 when individual RRV-injected group is compared to saline-injected control.
Injury to cholangiocytes and promotion of auto-recognition of duct epithelium by primed-CD8+ cells
To examine if neonatal CD8+ cells directly injure epithelial cells, we performed a detailed histological comparison of the bile duct epithelium in early phases after RRV challenge in CD4- or CD8-depleted mice and controls. The epithelium appeared normal in the first 2 days in all groups. By day 3, focal epithelial injury was easily identified in wild type and CD4-depleted mice, followed by mucosal sloughing and invasion of the lumen by inflammatory cells between 4-5 days (Fig. 5). In contrast, the epithelial lining of CD8-depleted mice appeared mostly intact up to 4 days, with only mild epithelial injury noted by 5 days but no luminal obstruction (Fig. 5 and Supplementary Table 3).
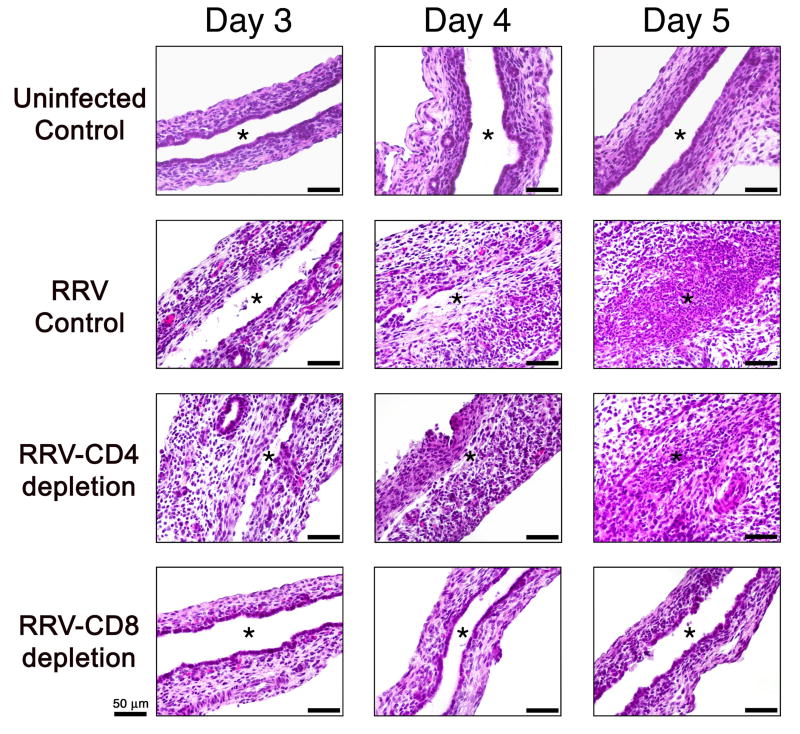
Figure 5. Loss of CD8+ cells decreases injury to bile duct epithelium.
Hematoxylin/eosin staining of longitudinal sections of extrahepatic bile ducts from uninfected controls and RRV challenged mice with or without depletion of CD4+ or CD8+ cells. An intact duct epithelium is present on days 3-5 in normal mice. In CD8-depleted mice, the epithelium also appears normal at day 3 and 4 and displays a mild focal epithelial injury at 5 days. In contrast, bile ducts of RRV-infected controls or CD4-depleted mice show extensive duct injury and obstruction at 4-5 days. Asterisk denotes the bile duct lumen.
Then, to directly determine whether CD8+ cells injure duct epithelium, we analyzed the cytolytic function of hepatic CD4+ and CD8+ cells in co-culture with a murine cholangiocyte cell line (mCL) infected with RRV.10, 15 While RRV-primed CD4+ and RRV-naïve CD8+ cells were unable to induce mCL cytolysis, RRV-primed-CD8+ cells promoted mCL lyses within 5 hours of culture (Fig. 6). The cytolytic properties were specific to mCL cells, as supported by the lack of lysis of the 67-NR breast cancer cell line in co-culture with RRV-primed CD8+ cells (Fig. 6). Notably, RRV-primed CD8+ cells also induced cytolysis in naïve mCL cells, implying that once primed in vivo the effector properties of CD8+ T cells can kill uninfected neighboring cholangiocytes.
Figure 6. RRV-primed hepatic CD8+ T cells induce cholangiocyte lysis.
51Cr release assays showing increased lysis of a murine cholangiocyte cell line (mCL) by CD8+ T cells isolated from newborn mice 7 days after RRV challenge, which increases several fold above baseline levels of CD8+ T cells from saline-injected (control) mice. The specificity of the findings is shown by the lack of lysis of the 67-NR breast cancer cell line by CD8+ or CD4+ cells and by the inability of RRV-primed CD4+ cells to promote lysis of mCL cells. Ratio represents mCL or 67-NR cells (as target cells) to CD8+ cells (as effector cells). Results are representative of two independent experiments, with hepatic lymphocytes obtained from a pool of 15-20 livers for each experiment.
To test the hypothesis that primed-CD8+ cells can positively modulate auto-recognition of the duct epithelium, we adoptively transferred intraperitoneally 8×105 CD8+ cells from livers of saline- or RRV-infected mice to neonatal Balb/c Rag2-/- mice. These mice were used as recipients because they are known to tolerate transfer of syngeneic lymphocytes and allow for the identification of transplanted cells. Similar transfer experiments using RRV-primed hepatic CD4+ cells served as controls. All cell aliquots were tested negative for RRV by virus titration (data not shown). We found very few or no CD3+ cells in bile ducts from mice transplanted with naïve CD8+ cells (Fig. 7A,B). Transfer of RRV-primed CD4+ cells resulted in the detection of CD3+ cells in the subepithelial space (i.e.: duct wall; 53±37 cells/duct), but none to rare cells along the epithelium (Fig. 7A,B). In contrast, after transfer of RRV-primed CD8+ cells, the number of CD3+ cells increased substantially juxtaposed or within the duct epithelium (58±28 cells/duct) as well as in the subepithelial space (230±42 cells/duct; Fig. 7A-C). Rare or no CD3-stained cells were found in livers, kidneys, and lungs of mice transplanted with either of the three cell types (Supplementary Table 4). These data are in keeping with the preferential auto-recognition of the biliary microenvironment by RRV-primed-CD8+ cells, which establish contact with the epithelium and populate the wall of extrahepatic bile ducts.
Figure 7. RRV-primed hepatic CD8+T cells have tropism to the biliary epithelium.
Immunostaining of extrahepatic bile ducts with anti-CD3 and anti-cytokeratin antibodies shows a greater number of CD3+ cells in the subepithelial space (A) and within the epithelium (B) in mice transplanted with RRV-primed hepatic CD8+ cells. C depicts the red/pink staining in CD3+ cells and green staining in cytokeratin+ duct epithelium. C1 is from wild-type mice infected with RRV; C2 is from Rag2-/- mice transplanted with RRV-naïve hepatic CD8+ cells; and C3 and 4 are from Rag2-/- mice transplanted with RRV-primed CD8+ cells. Magnification × 6000; *denotes P<0.05 when RRV-CD4+ or RRV-CD8+ cells are compared to RRV-naïve CD8+ controls; N=4 mice per group.
Discussion
Our findings provide evidence that neonatal CD8+ cells play a key effector role in pathogenesis of experimental biliary atresia. Despite the lack of pre-existing immunologic memory, CD8+ cells are able to activate a broad proinflammatory program in response to a viral insult upon exit from the intrauterine environment, injure extrahepatic bile ducts, and drive an experimental phenotype akin to a neonatal human disease: biliary atresia. The Th1-like molecular signature is consistent with the well-recognized ability of neonatal T lymphocytes to activate an adult-like response.24 Although the immune system of neonates differs from adults quantitatively and qualitatively, cytotoxic T lymphocytes (CTL) from neonatal mice have been shown to elicit competent CTL activity in response to strong Th1-cell inducing agents, such as DNA vaccines or oligonucleotides containing CpG motifs.3-5 Here, we show that this response can evolve and injure a target epithelium (bile duct) and disrupt a vital physiological process (bile flow) in response to neonatal challenge with a viral pathogen (rotavirus).
Rotavirus is one of the most frequent causes of childhood diarrhea worldwide, but its role in pathogenesis of biliary atresia in humans remains undefined. While a diarrheal phenotype is observed in mice infected during the first two weeks of life, rotavirus infection soon after birth can produce biliary injury and inflammatory obstruction, suggesting a unique role of the developing neonatal immune system in phenotype determination.11 In the experiments reported herein, we found that among the immune cells neonatal CD8+ T cells emerge as a key determinant of the biliary atresia phenotype, as they are necessary for induction of bile duct injury and obstruction in the early postnatal period. Interestingly, the findings that livers of CD8-depleted mice express Ifng following RRV challenge clearly demonstrate that Ifng is important but not sufficient to promote the biliary atresia phenotype in neonatal mice. Collectively, these data support the existence of a biological setting in which Ifng serves as a molecular signal that promotes the infiltration of injured bile ducts by inflammatory cells, with CD8+ T cells emerging as cellular effectors of luminal obstruction. Therefore, both Ifng and CD8+ T cells constitute two complementary mechanisms that act in concert to produce the atresia phenotype.
The ability of primed-CD8+ cells to recognize diseased and healthy duct epithelium provides new evidence of disordered immunity in pathogenesis of experimental biliary atresia. The long-held view that developmental and immunologic factors play key roles in pathogenesis of biliary atresia in humans is supported by the temporo-spatial features of bile duct injury in the immediate postnatal period6, 25 and mounting evidence of a proinflammatory response at the time of diagnosis in humans.8, 9 In experimental atresia, hepatic and splenic CD4+ cells from RRV-infected mice challenged with inactivated RRV or protein lysates in culture have been shown to be important sources of Ifng.12 While this is in keeping with the recognition of reactive epitopes by CD4+ cells, we found that the in vivo production of Ifng in response to RRV challenge is not lost by depletion of CD4+ cells, but continue to be produced by CD8+ cells along with increased liver expression of other Th1 cytokines. The potential role of T lymphocytes in bile duct injury, perhaps in an autoimmune fashion, was suggested previously by transplantation of CD3+ cells (probably containing CD4+, CD8+ and NK cell subpopulations) into adult SCID mice.12 In these experiments, transplanted cells homed into bile ducts and induced pericholangitis but no luminal obstruction. However, the contribution of individual CD3+ cell subpopulations to targeting the bile duct was not assessed. Here, we show that between CD3/CD4+ and CD3/CD8+ lymphocytes, the recognition of bile ducts is assigned primarily to CD8+ cells, as supported by a halt in disease progression and maintenance of luminal continuity of the bile duct in mice lacking CD8+ cells. The ability of RRV-primed CD8+ cells (but not RRV-primed CD4+ cells) to promote lysis of RRV-infected and naïve cholangiocytes and to home to the bile duct in non-infected newborn recipients is in keeping with a biological model in which CD8+ cells clear RRV-infected cholangiocytes and extend the epithelial injury by targeting adjacent naïve cholangiocytes, perhaps in an autoimmune fashion. While individual molecular mechanisms of cell-mediated injury and of homing are not yet defined, this experimental model appears suitable to future studies investigating candidate circuits such as those controlled by cytokines and integrins.
Autoimmunity has also been proposed as one of the pathogenic mechanisms in infants with biliary atresia based on a prominent tissue infiltration of lymphocytes, surface expression of self-MHC molecules, detection of auto-antibodies, and prevalent HLA haplotypes in specific populations.26 Although primed-CD8+ cells homed into the biliary epithelium following adoptive transfer in our experimental model, they did not induce the complete atresia phenotype of duct obstruction. It is tempting to speculate that the development of the atresia phenotype by transplanted cells requires a much larger number of activated CD8+ T cells or the synergy of other effector cells (such as NK cells) to obstruct the duct lumen. It is also possible that the lack of duct obstruction may result from apoptosis of transferred neonatal cells after transplantation into neonatal mice. This concept is supported by a previous report that the number of lymphocytes undergoing apoptosis is higher following transplantation into neonates than in adult recipients upon re-exposure to antigen.27 Formal answers to these questions are beyond the scope of this report, and will be obtained by future experiments in which CD8+ cells are co-transplanted with other components of the neonatal immune system (such as primed CD4+ and/or NK cells). These unknowns notwithstanding, our data elevate neonatal CD8+ cells to an effector cell position in the pathogenesis of biliary obstruction in experimental atresia, and as a potential therapeutic target to halt progression of disease in humans.
Supplementary Material
Dot plots of flow cytometric analyses after surface staining of hepatic mononuclear cells with anti-CD3, CD4, and NK antibodies show the low population of NK/CD3+ and NK/CD4+ cells in saline-control neonatal livers in the first 14 days of life. The number of these cells increases several fold after RRV challenge, with peaks between 5-7 days after viral challenge. The right upper quadrants contain the mean ± standard deviation for the total number of hepatic NK/CD3+ or NK/CD4+ cells. Each group contains 3 samples per time point.
Hepatic mononuclear cells were stained with either anti-CD4 antibodies (if they were harvested from livers of mice injected with anti-CD4 depleting antibodies – panel A) or anti-CD8 antibodies (CD8-depleted mice – panel B) and subjected to flow cytometric analyses. The top rows for each panel shows the number of CD4+ or CD8+ cells in wild-type (WT) mice not injected with depleting antibodies. The right upper quadrants contain the mean ± standard deviation for the total number of hepatic CD4+ or CD8+ cells. Each group contains 3 samples per time point. Days 1, 3, and 5 represent the number of days after RRV challenge.
The patterns of hepatic mRNA expression for IL-2, IL12p40, TNFalpha, Cxcl9, 10 and 11 after RRV challenge are maintained in livers of mice with the loss of either CD4+ or CD8+ T cells, with a decrease in the peak level of expression in IL-2 and Cxcl9 in CD4-depleted mice 7 days after RRV challenge. Levels of mRNA were determined by real-time PCR and expressed as a ratio to GAPDH. *=P<0.05 for CD4-dep and CD8-dep groups, ⊗=P<0.05 for WT and CD4-dep groups, and §=P<0.05 for WT and CD8-dep groups; WT=wild type. N=3 mice per group and at each time point.
Acknowledgments
This work was supported by the NIH grant DK64008 (to JAB), and by the Integrative Morphology Core of the Digestive Disease Research Development Center (DK064403). The authors thank Lisa McMillin for technical assistance and Dr. William Balistreri and Dr. Mitchell Cohen for the critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res. 2000;22:177–90. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 2.Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–71. doi: 10.3109/08830180009088503. [DOI] [PubMed] [Google Scholar]
- 3.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–8. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998;16:1675–82. doi: 10.1016/s0264-410x(98)00054-1. [DOI] [PubMed] [Google Scholar]
- 5.Hassett DE, Zhang J, Slifka M, Whitton JL. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J Virol. 2000;74:2620–7. doi: 10.1128/jvi.74.6.2620-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–92. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 7.Schon P, Tsuchiya K, Lenoir D, Mochizuki T, Guichard C, Takai S, Maiti AK, Nihei H, Weil J, Yokoyama T, Bouvagnet P. Identification, genomic organization, chromosomal mapping and mutation analysis of the human INV gene, the ortholog of a murine gene implicated in left-right axis development and biliary atresia. Hum Genet. 2002;110:157–65. doi: 10.1007/s00439-001-0655-5. [DOI] [PubMed] [Google Scholar]
- 8.Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Sneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1563–1659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 9.Mack CL, Tucker RM, Sokol RJ, Karrer FM, Kotzin BL, Whitington PF, Miller SD. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–9. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–9. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Mack CL, Tucker RM, Lu BR, Sokol RJ, Fontenot AP, Ueno Y, Gill RG. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology. 2006;44:1231–9. doi: 10.1002/hep.21366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Silva HD, Van Driel IR, La Gruta N, Toh BH, Gleeson PA. CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology. 1998;93:405–8. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeal MM, Rae MN, Ward RL. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71:8735–42. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mano Y, Ishii M, Kisara N, Kobayashi Y, Ueno Y, Kobayashi K, Hamada H, Toyota T. Duct formation by immortalized mouse cholangiocytes: an in vitro model for cholangiopathies. Lab Invest. 1998;78:1467–8. [PubMed] [Google Scholar]
- 16.Hodgson PD, Grant MD, Michalak TI. Perforin and Fas/Fas ligand-mediated cytotoxicity in acute and chronic woodchuck viral hepatitis. Clin Exp Immunol. 1999;118:63–70. doi: 10.1046/j.1365-2249.1999.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco MA, Greenberg HB. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–6. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 19.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–9. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh RR, Hahn BH, Sercarz EE. Neonatal peptide exposure can prime T cells and, upon subsequent immunization, induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–21. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–53. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 22.Chen N, Field EH. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–41. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 24.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 25.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57:87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–40. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dot plots of flow cytometric analyses after surface staining of hepatic mononuclear cells with anti-CD3, CD4, and NK antibodies show the low population of NK/CD3+ and NK/CD4+ cells in saline-control neonatal livers in the first 14 days of life. The number of these cells increases several fold after RRV challenge, with peaks between 5-7 days after viral challenge. The right upper quadrants contain the mean ± standard deviation for the total number of hepatic NK/CD3+ or NK/CD4+ cells. Each group contains 3 samples per time point.
Hepatic mononuclear cells were stained with either anti-CD4 antibodies (if they were harvested from livers of mice injected with anti-CD4 depleting antibodies – panel A) or anti-CD8 antibodies (CD8-depleted mice – panel B) and subjected to flow cytometric analyses. The top rows for each panel shows the number of CD4+ or CD8+ cells in wild-type (WT) mice not injected with depleting antibodies. The right upper quadrants contain the mean ± standard deviation for the total number of hepatic CD4+ or CD8+ cells. Each group contains 3 samples per time point. Days 1, 3, and 5 represent the number of days after RRV challenge.
The patterns of hepatic mRNA expression for IL-2, IL12p40, TNFalpha, Cxcl9, 10 and 11 after RRV challenge are maintained in livers of mice with the loss of either CD4+ or CD8+ T cells, with a decrease in the peak level of expression in IL-2 and Cxcl9 in CD4-depleted mice 7 days after RRV challenge. Levels of mRNA were determined by real-time PCR and expressed as a ratio to GAPDH. *=P<0.05 for CD4-dep and CD8-dep groups, ⊗=P<0.05 for WT and CD4-dep groups, and §=P<0.05 for WT and CD8-dep groups; WT=wild type. N=3 mice per group and at each time point.