Abstract
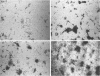
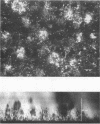
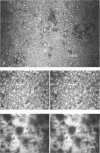
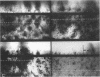
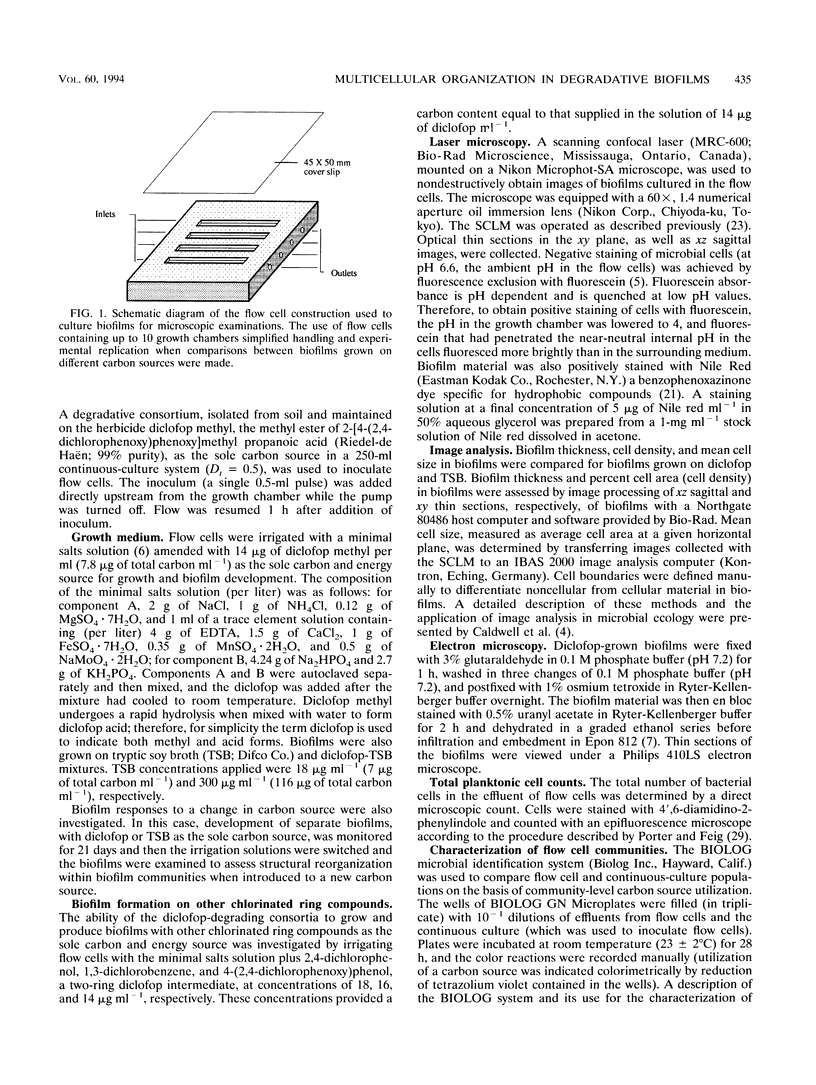
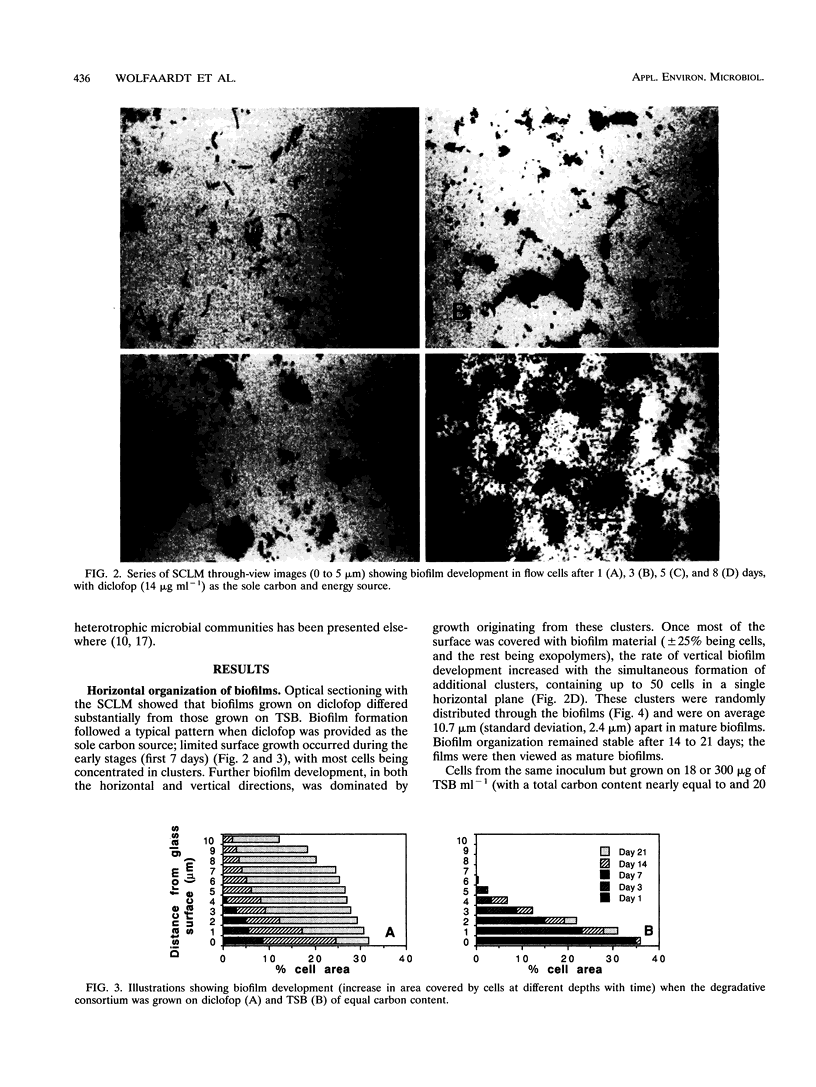
Diclofop methyl, a commercial herbicide, was used as the sole carbon source to cultivate diclofop-degrading biofilms in continuous-flow slide culture. The biofilms were analyzed by using scanning confocal laser microscopy and image analysis. Spatial relationships among members of the community were distinctive to diclofop-grown biofilms. These relationships did not develop when the biofilms were grown on more labile substrates but were conserved when the biofilms were cultivated with other chlorinated ring compounds. The structures included conical bacterial consortia rising to 30 μm above the surrounding biofilm, grape-like clusters of cocci embedded in a matrix of perpendicularly oriented bacilli, and other highly specific patterns of intra- and intergeneric cellular coaggregation and growth. These unique consortial relationships indicated that syntrophic interactions may be necessary for optimal degradation of diclofop methyl and other chlorinated ring compounds.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell D. E., Tiedje J. M. A morphological study of anaerobic bacteria from the hypolimnia of two Michigan lakes. Can J Microbiol. 1975 Mar;21(3):362–376. doi: 10.1139/m75-051. [DOI] [PubMed] [Google Scholar]
- Federle T. W., Hullar M. A., Livingston R. J., Meeter D. A., White D. C. Spatial distribution of biochemical parameters indicating biomass and community composition of microbial assemblies in estuarine mud flat sediments. Appl Environ Microbiol. 1983 Jan;45(1):58–63. doi: 10.1128/aem.45.1.58-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland J. L., Mills A. L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol. 1991 Aug;57(8):2351–2359. doi: 10.1128/aem.57.8.2351-2359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. M., Mallory L. M., Alexander M. Reasons for possible failure of inoculation to enhance biodegradation. Appl Environ Microbiol. 1985 Oct;50(4):977–983. doi: 10.1128/aem.50.4.977-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben W. E., Schroeter B. M., Calabrese V. G., Olsen R. H., Kukor J. K., Biederbeck V. O., Smith A. E., Tiedje J. M. Gene probe analysis of soil microbial populations selected by amendment with 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1992 Dec;58(12):3941–3948. doi: 10.1128/aem.58.12.3941-3948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. Mechanisms of bacterial degradation and transformation of chlorinated monoaromatic compounds. J Basic Microbiol. 1990;30(2):115–141. doi: 10.1002/jobm.3620300214. [DOI] [PubMed] [Google Scholar]
- Klingler J. M., Stowe R. P., Obenhuber D. C., Groves T. O., Mishra S. K., Pierson D. L. Evaluation of the Biolog automated microbial identification system. Appl Environ Microbiol. 1992 Jun;58(6):2089–2092. doi: 10.1128/aem.58.6.2089-2092.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Surface recognition among oral bacteria: multigeneric coaggregations and their mediators. Crit Rev Microbiol. 1989;17(2):137–159. doi: 10.3109/10408418909105746. [DOI] [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Hoyle B. D., Costerton J. W., Caldwell D. E. Optical sectioning of microbial biofilms. J Bacteriol. 1991 Oct;173(20):6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod F. A., Guiot S. R., Costerton J. W. Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microbiol. 1990 Jun;56(6):1598–1607. doi: 10.1128/aem.56.6.1598-1607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols P. D., Henson J. M., Guckert J. B., Nivens D. E., White D. C. Fourier transform-infrared spectroscopic methods for microbial ecology: analysis of bacteria, bacteria-polymer mixtures and biofilms. J Microbiol Methods. 1985;4:79–94. doi: 10.1016/0167-7012(85)90023-5. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Sjollema J., van der Mei H. C., Uyen H. M., Busscher H. J. Direct observations of cooperative effects in oral streptococcal adhesion to glass by analysis of the spatial arrangement of adhering bacteria. FEMS Microbiol Lett. 1990 Jun 1;57(3):263–269. doi: 10.1016/0378-1097(90)90078-5. [DOI] [PubMed] [Google Scholar]
- Smith A. E. Degradation of the herbicide dichlorfop-methyl in prairie soils. J Agric Food Chem. 1977 Jul-Aug;25(4):893–898. doi: 10.1021/jf60212a020. [DOI] [PubMed] [Google Scholar]
- Thiele Jurgen H., Chartrain M., Zeikus J. Gregory. Control of Interspecies Electron Flow during Anaerobic Digestion: Role of Floc Formation in Syntrophic Methanogenesis. Appl Environ Microbiol. 1988 Jan;54(1):10–19. doi: 10.1128/aem.54.1.10-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]