Abstract
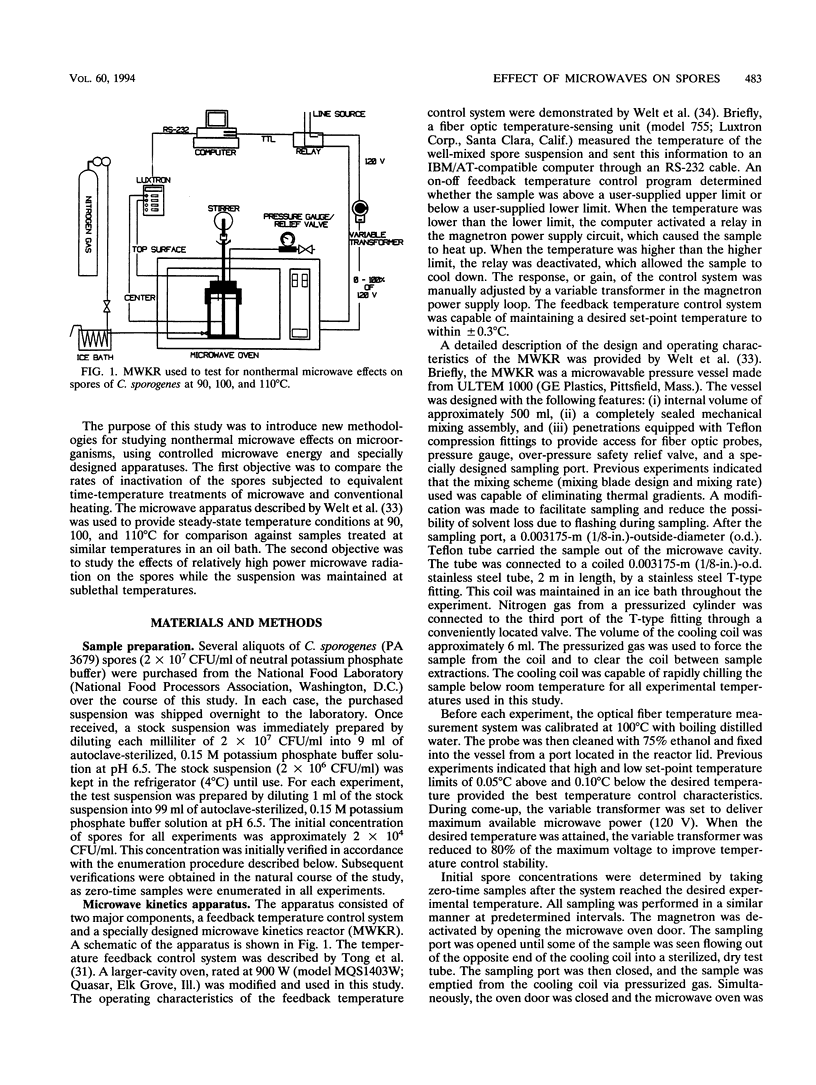
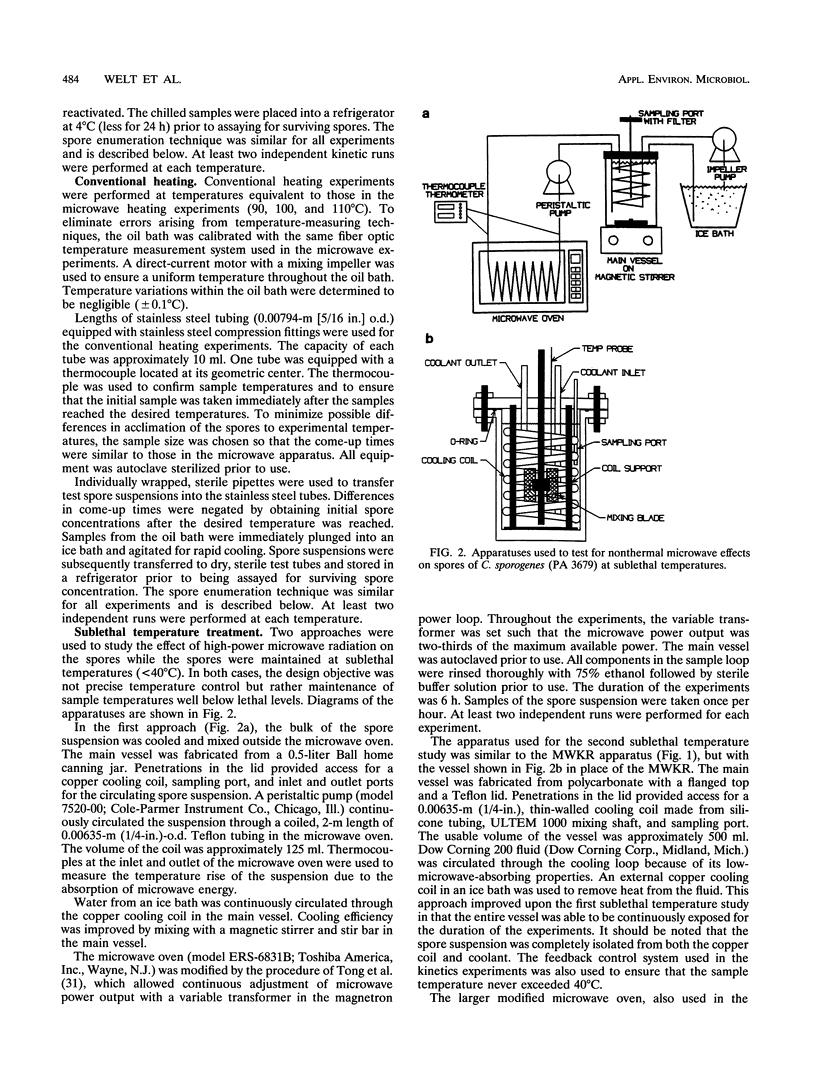
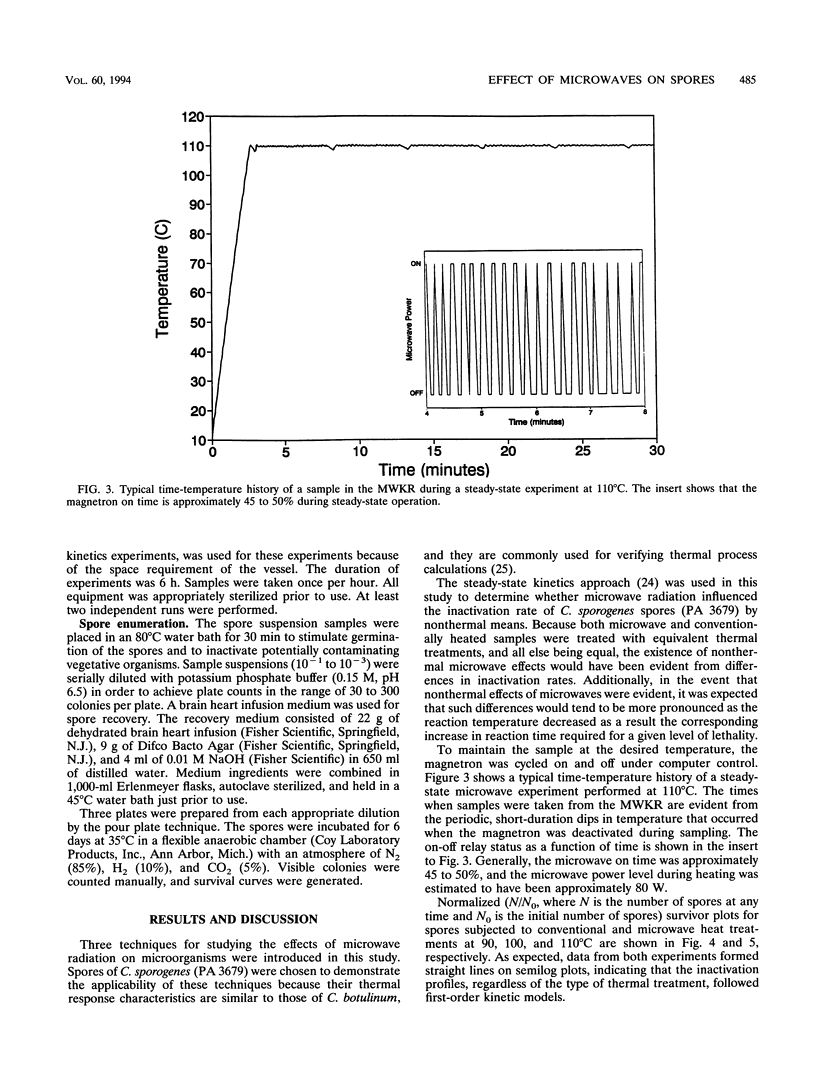
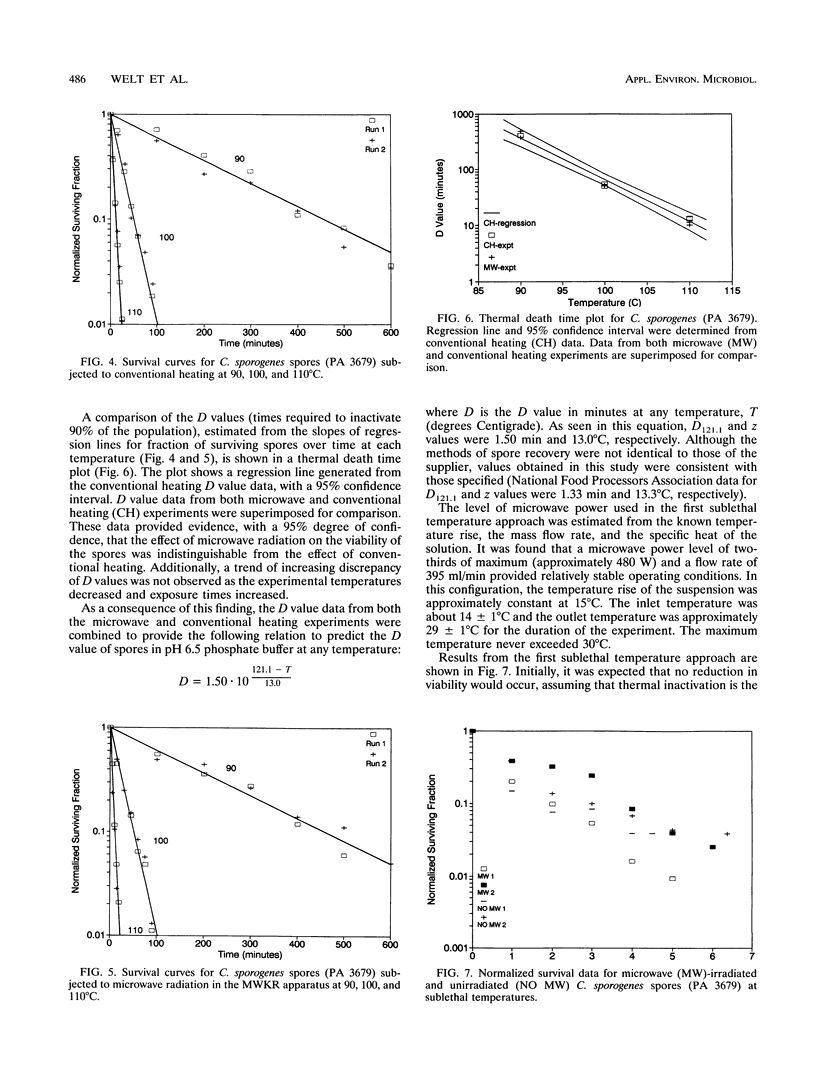
Three techniques for studying effects of microwave radiation on microorganisms were introduced. Spores of Clostridium sporogenes (PA 3679) were chosen as a test organism because the kinetic parameters for thermal inactivation are well known and because of the importance of the genus Clostridium to the food industry. For the first technique, a specially designed kinetics vessel was used to compare inactivation rates of microwave-heated and conventionally heated spores at steady-state temperatures of 90, 100, and 110 degrees C. Rates were found to be similar at the 95% confidence level. The second and third techniques were designed to study the effect of relatively high power microwave exposure at sublethal temperatures. In the second approach, the suspension was continuously cooled via direct contact with a copper cooling coil in a well-mixed vessel, outside the microwave oven. The suspension was pumped through a Teflon loop in the oven, where it continuously absorbed approximately 400 W of microwave power. Inactivation occurred in both irradiated and unirradiated samples. It was suspected that copper ions entered the suspension from the copper coil and were toxic to the spores. The fact that the results were similar, however, implied the absence of nonthermal microwave effects. In the third approach, the copper coil was replaced with a silicone tubing loop in a microwave transparent vessel. The suspension was continuously irradiated at 150 W of microwave power. No detectable inactivation occurred. Results indicated that the effect of microwave energy on viability of spores was indistinguishable from the effect of conventional heating.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cha J. S., Cooksey D. A. Copper Hypersensitivity and Uptake in Pseudomonas syringae Containing Cloned Components of the Copper Resistance Operon. Appl Environ Microbiol. 1993 May;59(5):1671–1674. doi: 10.1128/aem.59.5.1671-1674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipley J. R. Effects of microwave irradiation on microorganisms. Adv Appl Microbiol. 1980;26:129–145. doi: 10.1016/s0065-2164(08)70333-2. [DOI] [PubMed] [Google Scholar]
- Cooksey D. A., Azad H. R., Cha J. S., Lim C. K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl Environ Microbiol. 1990 Feb;56(2):431–435. doi: 10.1128/aem.56.2.431-435.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote P. J., Holyoak C. D., Cole M. B. Thermal inactivation of Listeria monocytogenes during a process simulating temperatures achieved during microwave heating. J Appl Bacteriol. 1991 Jun;70(6):489–494. doi: 10.1111/j.1365-2672.1991.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Cope F. W. Superconductivity--a possible mechanism for non-thermal biological effects of microwaves. J Microw Power. 1976 Sep;11(3):267–270. doi: 10.1080/00222739.1976.11689004. [DOI] [PubMed] [Google Scholar]
- Cross G. A., Fung D. Y. The effect of microwaves on nutrient value of foods. Crit Rev Food Sci Nutr. 1982;16(4):355–381. doi: 10.1080/10408398209527340. [DOI] [PubMed] [Google Scholar]
- DESSEL M. M., BOWERSOX E. M., JETER W. S. Bacteria in electronically cooked foods. J Am Diet Assoc. 1960 Sep;37:230–233. [PubMed] [Google Scholar]
- Dreyfuss M. S., Chipley J. R. Comparison of effects of sublethal microwave radiation and conventional heating on the metabolic activity of Staphylococcus aureus. Appl Environ Microbiol. 1980 Jan;39(1):13–16. doi: 10.1128/aem.39.1.13-16.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich H. The extraordinary dielectric properties of biological materials and the action of enzymes. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4211–4215. doi: 10.1073/pnas.72.11.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa H., Ushioda H., Kudo Y. Kinetics of Escherichia coli destruction by microwave irradiation. Appl Environ Microbiol. 1992 Mar;58(3):920–924. doi: 10.1128/aem.58.3.920-924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblith S. A. Basic principles of microwaves and recent developments. Adv Food Res. 1966;15:277–301. doi: 10.1016/s0065-2628(08)60082-8. [DOI] [PubMed] [Google Scholar]
- Goldblith S. A., Wang D. I. Effect of Microwaves on Escherichia coli and Bacillus subtilis. Appl Microbiol. 1967 Nov;15(6):1371–1375. doi: 10.1128/am.15.6.1371-1375.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALANT H. Physiological hazards of microwave radiation: a survey of published literature. Can Med Assoc J. 1959 Oct 1;81:575–582. [PMC free article] [PubMed] [Google Scholar]
- Lechowich R. V., Beuchat L. R., Fox K. I., Webster F. H. Procedure for evaluating the effects of 2,450-megahertz microwaves upon Streptococcus faecalis and Saccharomyces cerevisiae. Appl Microbiol. 1969 Jan;17(1):106–110. doi: 10.1128/am.17.1.106-110.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. D., Bhave M. R., Mercer J. F., Camakaris J., Lee B. T. Cloning and characterization of cutE, a gene involved in copper transport in Escherichia coli. J Bacteriol. 1991 Nov;173(21):6742–6748. doi: 10.1128/jb.173.21.6742-6748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayland J. R., Brannen J. P., Morris M. E. On the interdependence of thermal and electromagnetic effects in the response of Bacillus subtilis spores to microwave exposure. Radiat Res. 1977 Jul;71(1):251–258. [PubMed] [Google Scholar]



