Abstract
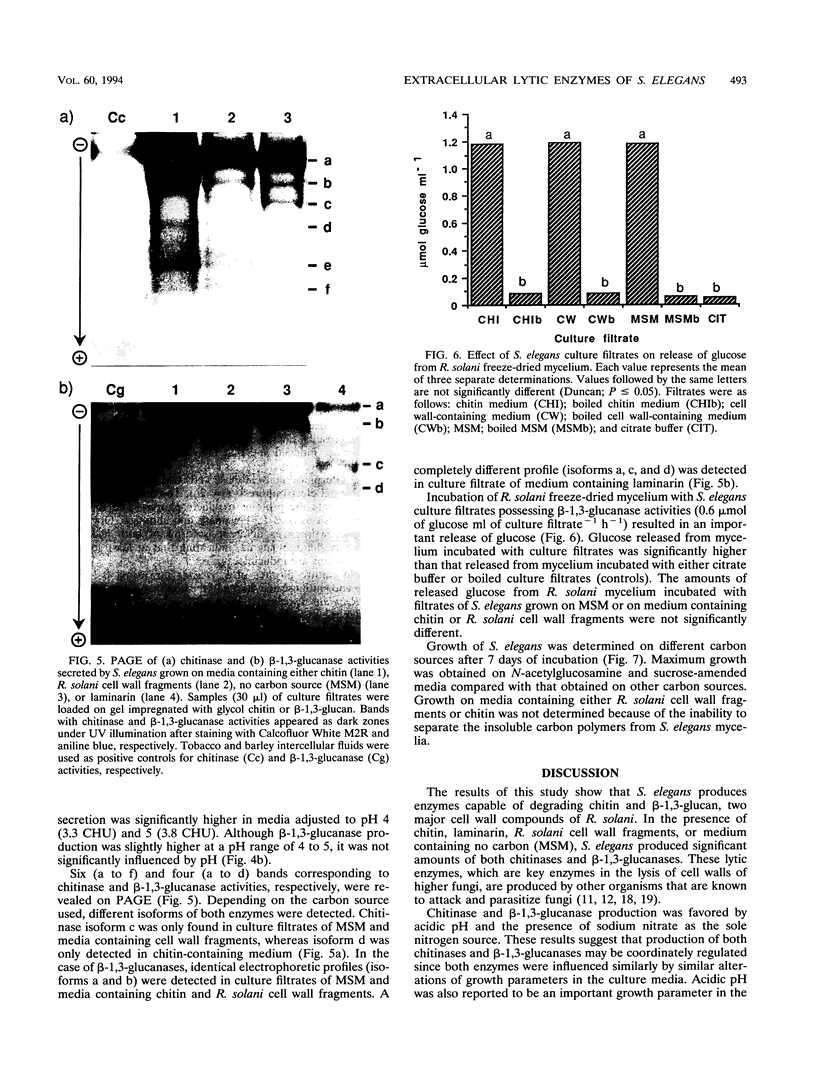
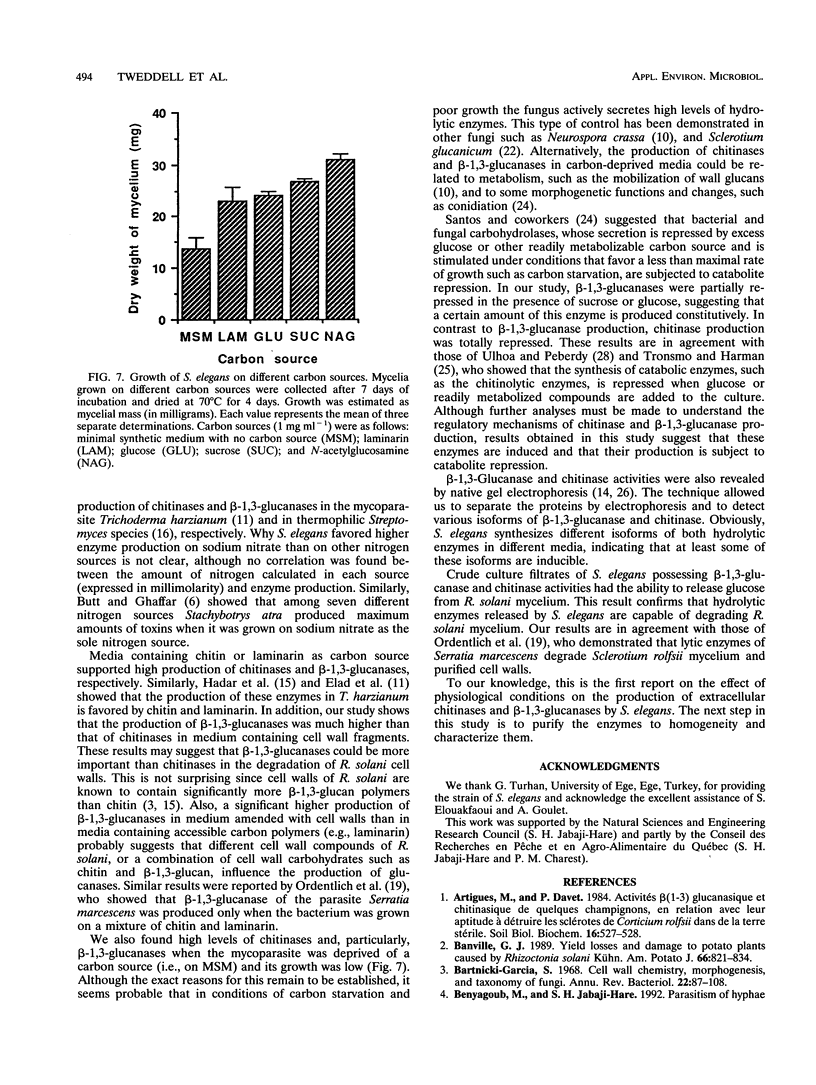
The in vitro production of chitinases and β-1,3-glucanases by Stachybotrys elegans, a mycoparasite of Rhizoctonia solani, was examined under various culture conditions, such as carbon and nitrogen sources, pH, and incubation period. Production of both enzymes was influenced by the carbon source incorporated into the medium and was stimulated by acidic pH and NaNO3. The activity of both enzymes was very low in culture filtrates from cells grown on glucose and sucrose compared with that detected on chitin (for chitinases) and cell wall fragments (for β-1,3-glucanases). Protein electrophoresis revealed that, depending on the carbon source used, different isoforms of chitinases and β-1,3-glucanases were detected. S. elegans culture filtrates, possessing β-1,3-glucanase and chitinase activities, were capable of degrading R. solani mycelium.
Full text
PDF
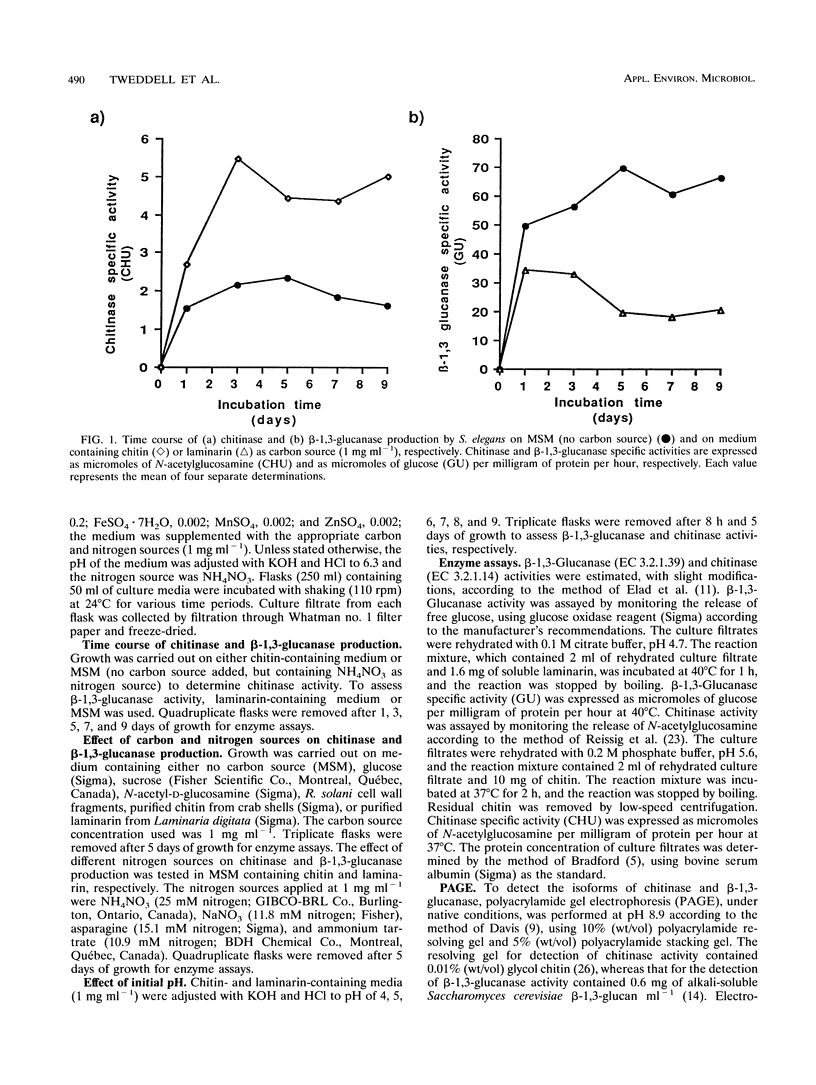
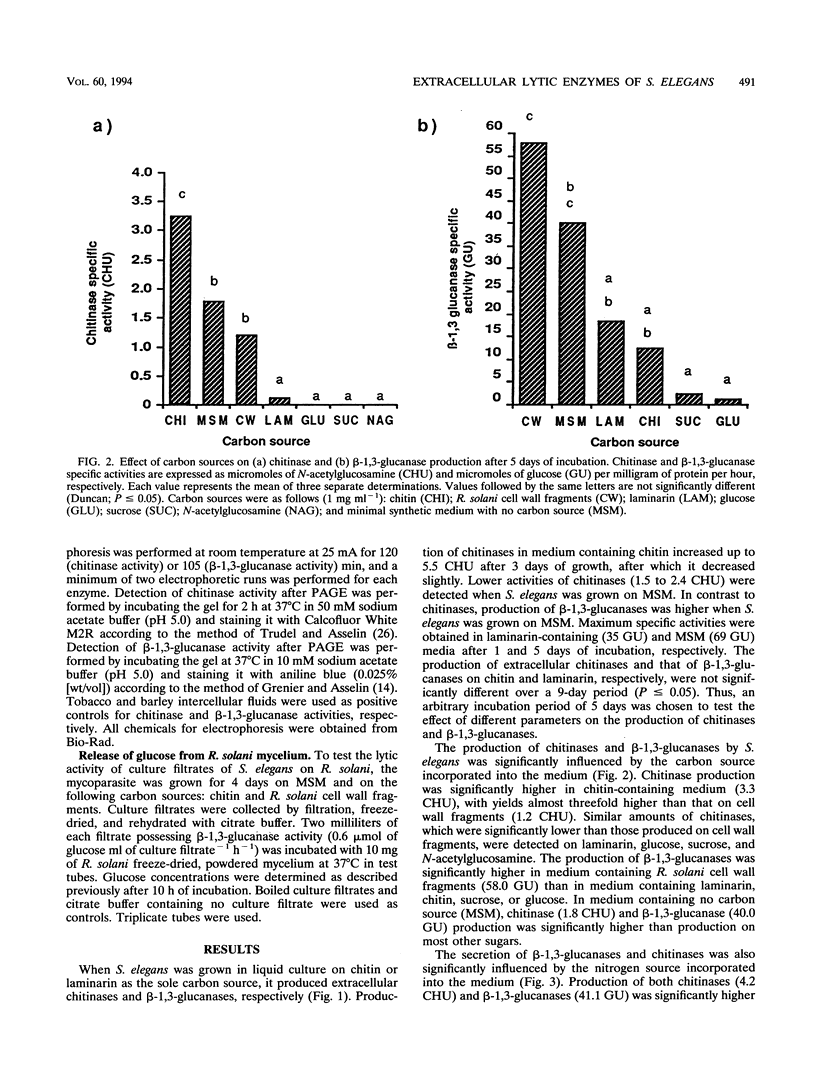
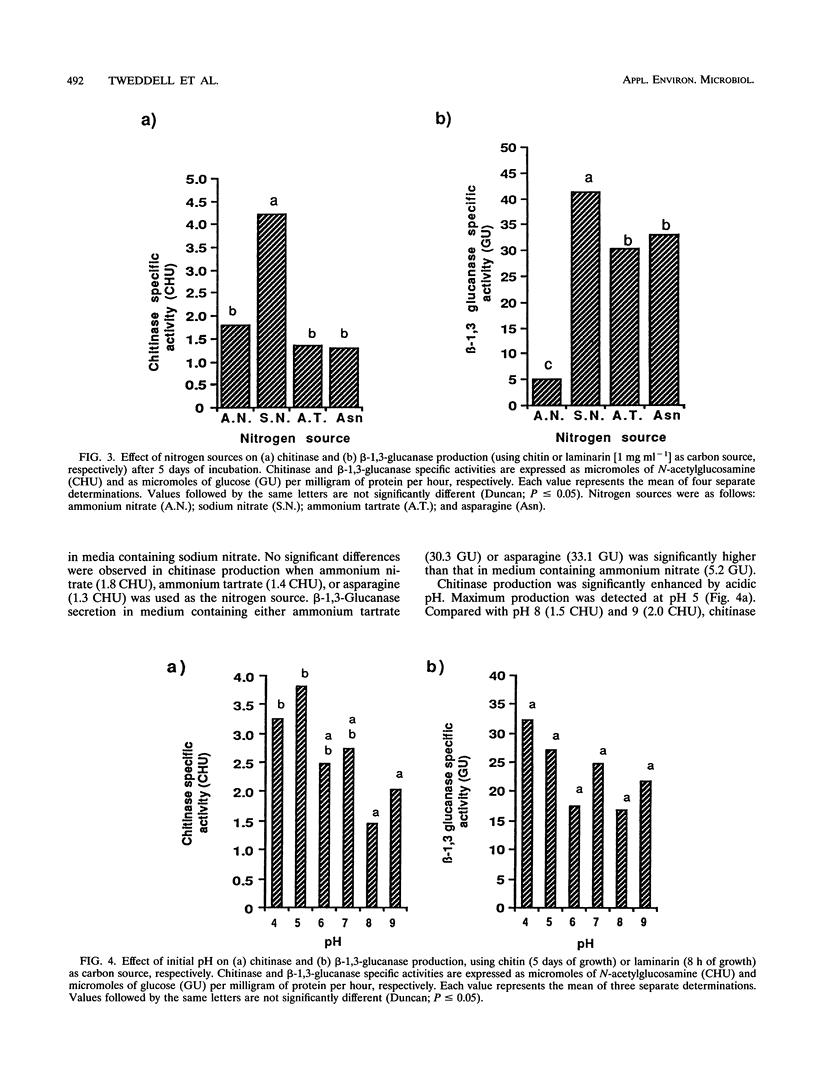

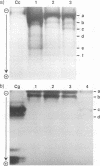
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chet I., Henis Y., Mitchell R. Chemical composition of hyphal and sclerotial walls of Sclerotium rolfsii Sacc. Can J Microbiol. 1967 Feb;13(2):137–141. doi: 10.1139/m67-019. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Lilley G., Bull A. T. The production of beta-1,3 glucanase by a thermophilic species of streptomyces. J Gen Microbiol. 1974 Jul;83(0):123–133. doi: 10.1099/00221287-83-1-123. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Santos T., Villanueva J. R., Nombela C. Production and catabolite repression of Penicillium italicum beta-glucanases. J Bacteriol. 1977 Jan;129(1):52–58. doi: 10.1128/jb.129.1.52-58.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel J., Asselin A. Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem. 1989 May 1;178(2):362–366. doi: 10.1016/0003-2697(89)90653-2. [DOI] [PubMed] [Google Scholar]
- Ulhoa C. J., Peberdy J. F. Regulation of chitinase synthesis in Trichoderma harzianum. J Gen Microbiol. 1991 Sep;137(9):2163–2169. doi: 10.1099/00221287-137-9-2163. [DOI] [PubMed] [Google Scholar]