Abstract
The presence of the P2Y2 (P2U-purinergic) receptor on the apical surface of airway tissue raises the possibility that aerosolized UTP might be used therapeutically to induce Cl− secretion in individuals with cystic fibrosis. However, the duration of the effects of UTP may be limited by enzymatic degradation. We therefore have analyzed the metabolism of UTP and its metabolite UDP on polarized human nasal epithelium (HNE), and have compared the pharmacological activities of these two uridine nucleotides. HPLC analysis of medium bathing the mucosal surface of HNE cells revealed the presence of an ecto-nucleotidase(s) that hydrolyzed [3H]UTP and [3H]UDP with t½ values (at 1 μM nucleotide) of 14 and 27 min, respectively. An ecto-nucleoside diphosphokinase activity also was observed, which promoted conversion of [3H]UDP into [3H]UTP in the presence of ATP. The effects of UDP on [3H]inositol phosphate accumulation, intracellular calcium levels ([Ca2+]i), and Cl− secretory rates (ICl−) were quantitated in HNE cells in the presence of hexokinase and glucose to ensure that no UTP (or ATP) contaminated UDP solutions during the assays. Although UDP does not activate the human P2Y2 receptor, mucosal addition of UDP promoted [3H]inositol phosphate accumulation with an EC50 of 190 nM. Mucosal addition of UTP stimulated [3H]inositol phosphate accumulation with an EC50 of 280 nM. The maximal effects of mucosal UDP on [3H]inositol phosphate, [Ca2+]i, and ICl− responses were approximately one-half of those observed with mucosal UTP. Serosal application of UTP promoted a 50% greater [3H]inositol phosphate and calcium response than did mucosal application of UTP. In contrast, UDP had no effect when added to the serosal medium. Repetitive mucosal applications of UDP to HNE cells resulted in a progressive loss, i.e., desensitization, of the [Ca2+]i and ICl− response to UDP, whereas the corresponding responses to UTP remained unchanged. Our results provide evidence for the existence of a UDP receptor on HNE cells that is pharmacologically distinct from the P2Y2 receptor. The relative stability of UDP on the airway surface and the apparent predominant mucosal expression of this putative UDP receptor make it a potential target for cystic fibrosis treatment.
Extracellular ATP regulates physiological responses in many tissues and cell types through a multi-gene family of P2 receptors (1–4). The existence of receptors that selectively recognize pyrimidines was proposed more than a decade ago (see ref. 5). However, support for the idea of specific pyrimidinergic receptors was lessened by the fact that the stimulatory activity of UTP in many tissues was mirrored by similar effects of ATP and by the eventual cloning of the P2Y2 (P2U-purinergic) receptor, which is equipotently activated by the purine ATP and the pyrimidine UTP. Nonetheless, a novel receptor was identified on C6–2B rat glioma cells that is activated by UDP but not by adenine nucleotides (6), and two recently cloned G protein-coupled P2Y receptors, the P2Y4 and P2Y6 receptors, show high selectivity for uridine nucleotides and are activated only weakly or not at all by adenine nucleotides (7–10). The P2Y6 receptor, which is potently and selectively activated by UDP, subsequently was shown to be expressed endogeneously by C6–2B cells (11). Although mRNA for the P2Y6 receptor was detected in several tissues, including aortic smooth muscle cells, spleen, kidney, stomach, lung, intestine, and heart (7, 8), functional evidence for uridine nucleotide selective receptors in native tissues is limited.
Uridine nucleotide analogues that might discriminate between uridine nucleotide-selective receptors and the widely expressed P2Y2 receptor have not been available. The instability of most nucleotides in aqueous solution and metabolic interconversion of UTP and UDP during incubation with cells contribute additional problems. For example, we (12) and others (13) initially reported that UDP was a full agonist at the P2Y2 receptor, and Communi et al. (8) reported that UDP was a full and potent agonist at the P2Y4 receptor. However, we recently have demonstrated that UTP-free UDP is essentially inactive at both receptor types (11). Conversely, while Chang et al. (7) originally reported that UTP was the most potent agonist at the P2Y6 receptor, we have observed that UDP is at least 100-fold more potent than UTP (11), and the initially reported effects of UTP were likely due to contamination with UDP or formation of UDP during incubation with cells.
In this study we have adopted conditions that allow for examination of the biological effects of UDP on primary cultures of polarized human nasal epithelial cells. We report that UDP potently stimulates formation of inositol phosphates, calcium mobilization, and chloride secretion in airway epithelial cells, and that the effects of UDP are not explained by activation of a P2Y2 receptor. The response to UDP is restricted to the mucosal surface of these human airway cells.
MATERIALS AND METHODS
Cell Cultures.
Human nasal epithelial cells were harvested from turbinates (protease 14) (Sigma) for 24–48 h at 4°C, as previously described (14). Cytosolic Ca2+ and inositol phosphate measurements were made on nasal cell monolayers plated on porous Transwell Col filters (pore diameter 0.45 μm; Costar) and maintained in Ham’s F-12 medium supplemented with 10 ng/ml epidermal growth factor, 3.75 ng/ml endothelial cell growth factor, 500 ng/ml hydrocortisone, 5 ng/ml insulin, and 1 mM CaCl2 (F12 + 4X medium). Assays were carried out 7–10 days after seeding, a time coincident with the development of the maximal transepithelial potential difference (16).
Measurement of Inositol Phosphates.
Confluent cells were labeled for 18 h in inositol-free DMEM containing 4.5 g/liter glucose and 5 μCi/ml [3H]myo-inositol (1 Ci = 37 GBq). The cells were preincubated with 10 mM LiCl and challenged with agonists for an additional 10 min. No changes of medium were made subsequent to the addition of [3H]myo-inositol to avoid release of endogenous ATP from stressed cells (12). Incubations were terminated by the addition of 5% ice-cold trichloroacetic acid and the resultant [3H]inositol phosphates were separated on Dowex AG1-X8 columns as described (15).
Combined Bioelectric and Cytosolic Ca2+ Measurements.
Nasal cells grown on Transwell Col filters affixed to O-rings were maintained in F12 + 4X medium and studied 7–10 days after cell plating as described above. For Ca2+i measurements, cells were loaded with Fura-2 and mounted in a miniature Ussing chamber over an objective of a microscope (Zeiss) coupled to a microfluorimeter, and cytosolic Ca2+i levels were quantitated as described (15). The fluorescence intensity ratio (excitation 340/380; emission ≥450 nm) was collected from a field of 30–40 nasal cells on monolayers and converted to Ca2+ concentration as described (17). For simultaneous measurements of Cl− secretion, the transepithelial potential difference was measured by a Voltage-Clamp/Pulse Generator (Model VCC600, Physiologic Instruments, San Diego) and recorded on a two-channel recorder (Linsesis model L200S). To calculate changes in Cl− secretory current (ΔICl−), a defined 1-s current pulse was delivered across the monolayer every 10 s. The nasal tissue was converted from its native Na+ absorptive state to a Cl− secretory state by exposing monolayers to a luminal medium containing 0 Na+/low Cl− and to a basolateral medium of Krebs bicarbonate Ringer solution.
Hydrolysis of [3H]UTP and [3H]UDP.
Nasal monolayers were grown to confluence on 6.5-mm transwells (Costar) as indicated above. The cells were washed twice and preincubated with 300 μl of Hepes-buffered (pH 7.4) DMEM medium for at least 1 h prior to the addition of the [3H] nucleotides to ensure that any endogeneously released ATP was degraded. Incubations were initiated by the addition of 1 μM [3H]UTP or [3H]UDP (0.5 μCi each) to either cell surface. Incubations were terminated at the indicated times by transferring the medium to a tube containing 30 μl of 50 mM EDTA and boiling for 2 min.
Nucleoside Diphosphokinase Assay.
To assay for the presence of ecto-nucleoside diphosphokinase activity, cells were incubated with [3H]UDP as detailed above and ATP (100 μM) was included in the incubation medium. The ATP-dependent conversion of [3H]UDP to [3H]UTP was quantitated as described (18).
HPLC Analysis.
Nucleotides were separated by HPLC (Shimadzu) via a strong anion exchange column (Rainin Instruments) (18) using a two-solvent system consisting of buffer A (45 mM ammonium formate, pH 4.6) and buffer B (250 mM sodium phosphate, pH 2.7). A linear gradient was developed from 100% buffer A to 100% B during the first 25 min. The column then was eluted with 100% buffer B for the following 15 min and with 100% buffer A for an additional 15 min from 45 to 60 min. Absorbance at 260 nm was monitored with an LSA UV detector (Shimadzu), and radioactivity was monitored online with a Flo-One detector (Packard). [3H]Nucleotides and [3H]nucleosides were quantitated as described (18).
Enzymatic Conversion of UTP to UDP.
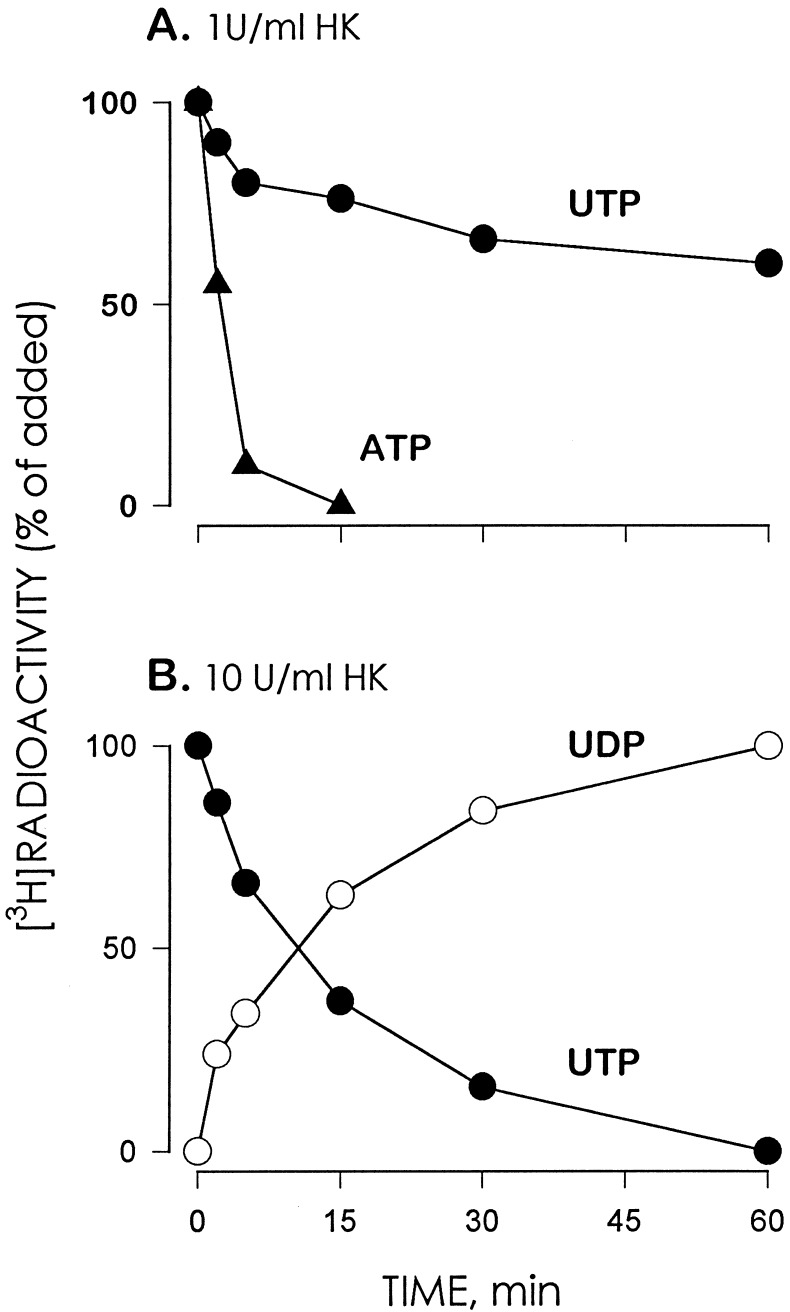
Accurate delineation of the pharmacological selectivities of nucleotide receptors is hampered by impurities frequently present in nucleotide solutions and by potential ecto-enzyme-catalyzed interconversion between triphospho- and diphosphonucleotides. Commercial preparations of UDP contain up to 2% UTP. Therefore, we have taken advantage of the enzymatic activity of hexokinase, which in the presence of glucose transfers the γ-phosphate of ATP to glucose producing ADP and glucose 6-phosphate as products (19). Although ATP is the preferential substrate for hexokinase, other nucleoside triphosphates also serve as γ-phosphate donors although at slower rates of reaction (19). Hexokinase (1 unit/ml) hydrolyzed UTP at a 60- to 80-fold slower rate than the hydrolysis of ATP (Fig. 1A). However, incubation of 1 mM UTP with 10 units/ml hexokinase resulted in quantitative conversion of UTP to UDP (Fig. 1B). To ensure that no UTP contaminated UDP, we routinely preincubated stock solutions of UDP (1 mM) with 10 units/ml hexokinase and 25 mM glucose for 1 h prior to assays.
Figure 1.
Hexokinase (HK) catalyzes the conversion of [3H]UTP to [3H]UDP. (A) Time course for ATP and UTP hydrolysis in the presence of 1 unit/ml HK. Incubations were carried out at 37°C in 1 ml Hepes-buffered DMEM (pH 7.4) containing 25 mM glucose and 1 mM (0.5 μCi) of either [3H]ATP or [3H]UTP. At the times indicated, 5 mM EDTA was added to the samples followed by boiling. (B) Time course for the conversion of [3H]UTP to [3H]UDP in the presence of 10 units/ml HK. Incubations were as indicated above except for the HK concentration. The [3H]species were resolved by HPLC as detailed. The results are expressed as the percent conversion of [3H]nucleotide triphosphate to [3H]nucleotide diphosphate. Data indicate the mean value of one experiment representative of two independent experiments performed with duplicate samples that differed by less than 20%.
Synthesis of [3H]UDP.
[3H]UDP was prepared by incubating [3H]UTP with hexokinase and glucose followed by boiling for 2 min, as indicated above.
Materials.
UTP and ATP were obtained from Pharmacia, and UDP and hexokinase were from Boehringer Mannheim. [3H]myo-inositol (20 Ci/mmol) was from ARC (St. Louis) and [3H]UTP and [3H]ATP (97% and 99% pure, respectively) (17–20 Ci/mmol) were from Amersham.
RESULTS
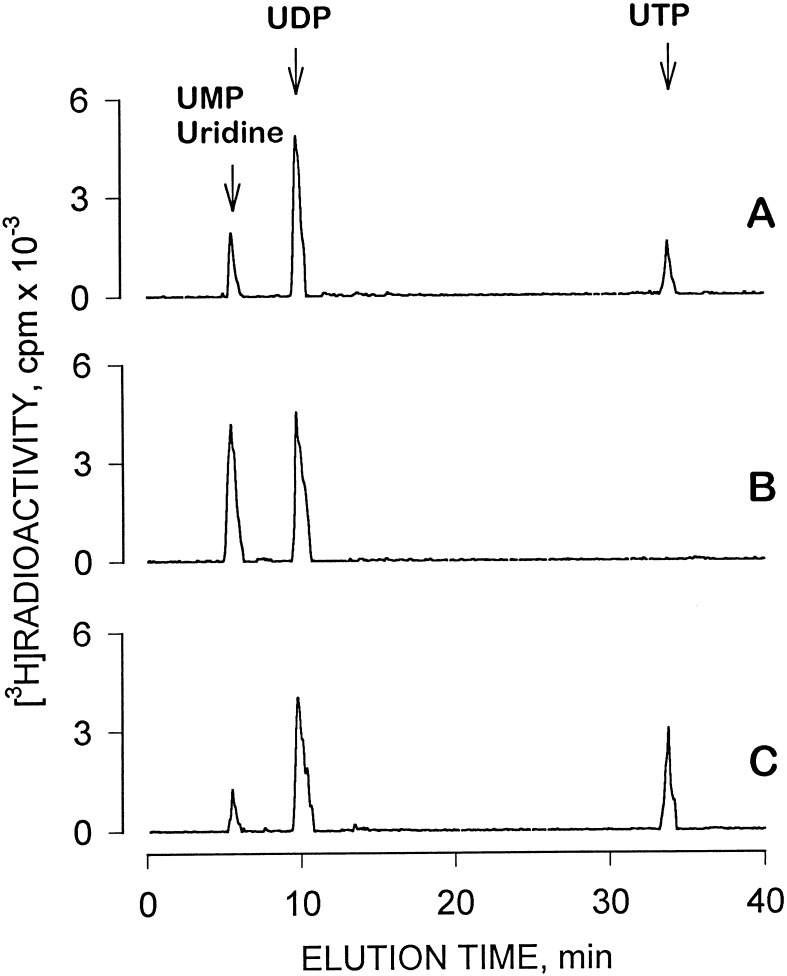
The metabolism of [3H]UDP and [3H]UTP by human airway epithelial cell surfaces was examined. Incubation of 1 μM [3H]UTP for 20 min on the mucosal surface resulted in 73 ± 16% hydrolysis indicating the presence of ecto-nucleotidases and/or phosphatases. [3H]UDP accumulated as the major breakdown product of [3H]UTP (Fig. 2A). Mucosal [3H]UDP (1 μM) also was hydrolyzed, although to a lesser extent than [3H]UTP (Fig. 2B). Time course experiments indicated that the half-life (t½) values for mucosal [3H]UTP and [3H]UDP were 14 ± 2 min and 27 ± 3 min, respectively (not shown). Hydrolysis of both [3H]UTP and [3H]UDP was determined on both cell surfaces and the data are summarized in Table 1.
Figure 2.
Metabolism of [3H]UTP and [3H]UDP by human nasal epithelial cells. Confluent polarized cells were incubated for 20 min at 37°C in the presence of 1 μM (0.2 μCi) of [3H]UTP (A), [3H]UDP (B), or [3H]UDP combined with 100 μM ATP (C). All additions were to the mucosal bath (final volume, 300 μl). Incubations were terminated by transferring the mucosal medium to an Eppendorf tube containing 30 μl of 50 mM EDTA followed by boiling. [3H]Species were separated by HPLC as described. The traces are representative of at least three independent experiments performed with duplicates.
Table 1.
Hydrolysis of [3H]UTP and [3H]UDP by the mucosal and serosal surfaces of human nasal epithelial cells
| Nucleotide hydrolysis, pmol/transwell
|
||
|---|---|---|
| Mucosal | Serosal | |
| [3H]UTP | 147 ± 22 | 98 ± 11 |
| [3H]UDP | 87 ± 9 | 70 ± 9 |
Primary cultures of human nasal epithelium cells were grown to confluence on 6.5-mm transwells as a polarized epithelium. The cells were washed twice with prewarmed DMEM–Hepes medium (pH 7.4), and were pre-incubated for 1 h at 37°C with 0.5 ml of medium added on each side. Incubations were initiated by the addition 50 μl of 10 μM (0.5 μCi) [3H]UTP or [3H]UDP on the indicated cell surface. Medium samples were collected after a 20-min incubation period and analyzed by HPLC as described. The values represent the mean (± range from the mean) from two experiments performed with duplicate samples.
Nucleoside diphosphokinase catalyzes the transfer of the γ-phosphate from nucleoside triphosphates to nucleoside diphosphates. We previously have observed the presence of a nucleoside diphosphokinase activity on the surface of 1321N1 human astrocytoma cells, which converts UDP (and ADP) to UTP (or ATP) and confounds analyses of the pharmacological effects of diphosphonucleotides. To examine the potential presence of ecto-nucleoside diphosphokinase activity in airway epithelial cells, [3H]UDP was added to the mucosal surface in combination with ATP (Fig. 2C). [3H]UTP was rapidly formed under these conditions, and similar results were obtained with either 0.1 μM or 100 μM [3H]UDP. Comparable results also were obtained in experiments examining nucleoside diphosphokinase activity on the serosal side (data not shown). Since potential ATP release from epithelial cells during tissue manipulations and consequential phosphorylation of UDP to UTP by nucleoside diphosphokinase may complicate study of the actions of UDP, hexokinase (2 units/ml) and glucose were included in all subsequent assays examining the effects of UDP (see Materials and Methods and Fig. 1). No accumulation of [3H]UTP occurred under these incubation conditions (not shown).
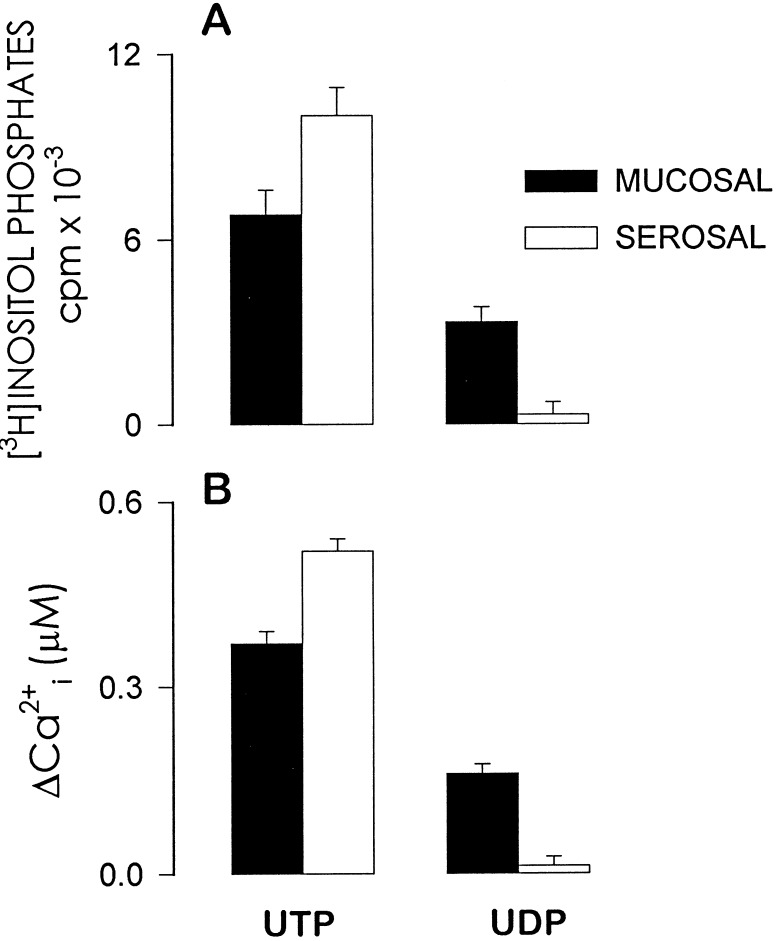
Primary cultures of polarized human airway epithelial cells express P2Y2 receptors on both cell surfaces (20). We have reported that functional expression of the P2Y2 receptor in polarized human nasal epithelial cells is asymmetric and that the receptor apparently couples more efficaciously to its effector phospholipase C on the serosal (basolateral) surface than on the mucosal (apical) surface (15). Consistent with this previous study, UTP (100 μM) promoted larger [3H]inositol phosphate and calcium responses when applied to the basolateral bath of polarized primary human nasal epithelial cells than when applied to the mucosal bath (Fig. 3). The Cl− secretory responses (ΔICl−) following addition of 100 μM UTP to either the mucosal or the serosal bath were 74 ± 11 μA/cm2 and 34 ± 3 μA/cm2, respectively. Application of UDP to the serosal bath had negligible effect on inositol phosphate accumulation. In contrast, mucosal UDP (100 μM) promoted marked accumulation of [3H]inositol phosphates and calcium mobilization, and the maximal effects of UDP were approximately one-half the magnitude of the responses observed with mucosal UTP (Figs. 3 and 4). Similarly, mucosal but not serosal UDP stimulated Cl− secretion (ΔICl− = −16 ± 3 μA·cm2 for 3 μM mucosal UDP; ΔICl− = 0 ± 0 μA·cm2 for 3 μM serosal UDP) and the magnitude of this response was approximately one-half of the mucosal UTP response (Fig. 4). The effects of mucosal application of UDP cannot be explained by activation of P2Y2 receptors, since UTP-free UDP is essentially inactive at the P2Y2 receptor (11) and because UDP caused little or no effect when applied to the P2Y2 receptor-expressing serosal side of the cell monolayers (Figs. 3 and 4). The larger mucosal versus serosal effect of UDP contrasts with the predominantly basolateral effects of UTP (Fig. 3) and ATP (15). Thus, the action of UDP in polarized airway epithelial cells does not coincide with that observed with P2Y2 receptor agonists (Figs. 3 and 4, and ref. 11)
Figure 3.
Sidedness of UTP and UDP effects on [3H]inositol phosphate formation and intracellular calcium mobilization in polarized human nasal epithelial cells. Confluent cells were loaded with [3H]myo-inositol and preincubated with LiCl (A) or with Fura-2 (B) as described. The cells were challenged with 100 μM of the indicated nucleotide, added to either the serosal or the mucosal medium. The results represent the mean (±SEM) from three experiments performed with triplicates (A) or from 14 individual experiments (B).
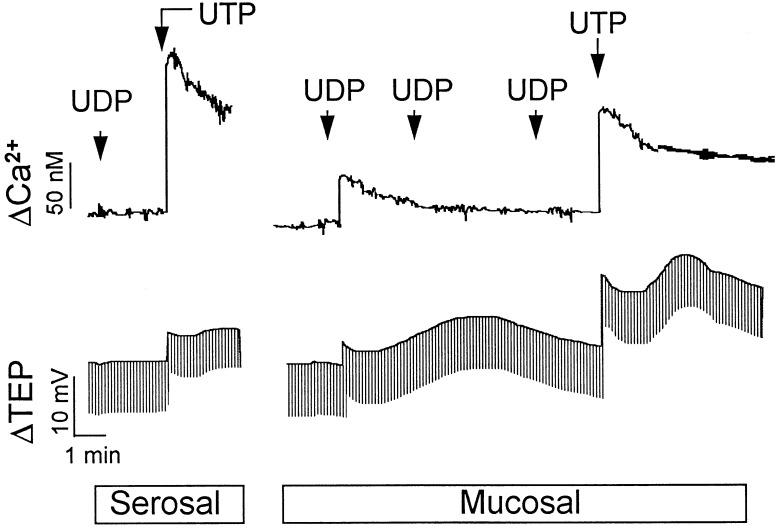
Figure 4.
Representative tracings of UDP- and UTP-promoted changes in intracellular Ca2+ concentration and Cl− diffusion potentials in human nasal epithelial cells. Primary cultures of human nasal epithelial cells were mounted on modified Ussing chambers as described. Changes in Ca2+ concentration (top tracings) or in Cl− secretion (bottom tracings) were simultaneously recorded after the addition to the basolateral medium of 100 μM UDP followed by 100 μM UTP (serosal), or after two consecutive additions of 3 μM UDP and then 10 μM UDP to the apical medium followed by the ipsilateral addition of 10 μM UTP (mucosal). Data are representative of at least eight independent experiments.
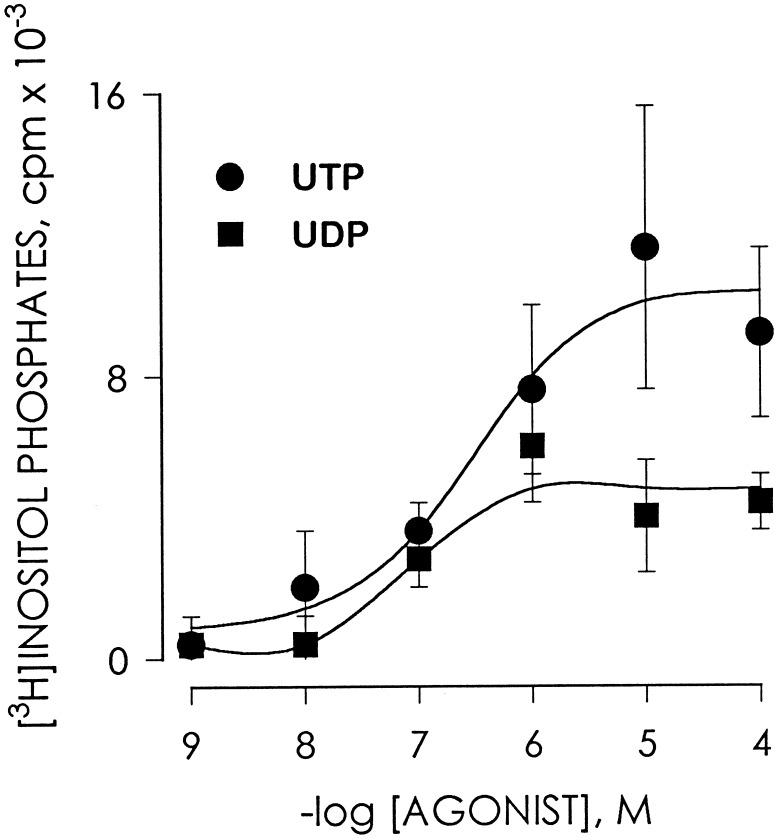
Although mucosal UDP was less efficacious than mucosal UTP in stimulating inositol phosphate formation in human nasal epithelium, UDP (EC50 = 190 ± 27 nM) and UTP (EC50 = 280 ± 35 nM) exhibited similar potencies (Fig. 5). Cross-desensitization experiments were carried out to further address the hypothesis that UDP promotes signaling responses in airway cells through a receptor distinct from the P2Y2 receptor (Fig. 4). Addition of 3 μM of UDP to the mucosal bath promoted mobilization of intracellular calcium with a ΔCa2+ of 66 ± 12 nM, and changes in Cl− secretory responses (ΔICl−) of −16.3 ± 3 μA/cm2 (n = 8). Subsequent addition of 3 μM UDP followed by 10 μM UDP did not result in elevation of intracellular Ca2+ (ΔCa2+ = 0) or in Cl− secretion (ΔICl− = 0), which suggests the occurrence of UDP-induced desensitization (Fig. 4). In contrast, responses to UTP [ΔCa2+ = 374 ± 24 nM (n = 19); ΔICl− = −74 ± 11 μA/cm2 (n = 12)] were retained in cells that were pretreated with UDP [ΔCa2+ = 310 ± 35 nM (n = 8); ΔICl− = −64 ± 10 μA/cm2 (n = 8)] (Fig. 4). Responses to ATP also were retained following multiple additions of UDP (not shown).
Figure 5.
Concentration-response relationship for mucosal UDP- and UTP-stimulated [3H]inositol phosphate formation in human nasal epithelial cells. Confluent cells were labeled with [3H]myo-inositol, preincubated with LiCl as described, and subsequently challenged for 10 min with the indicated concentration of UDP or UTP added to the mucosal medium. Data represent the mean value (±SEM) from three independent experiments performed with triplicate samples.
DISCUSSION
Existence of a G protein-coupled receptor that selectively recognizes UDP was originally established in studies of a receptor natively expressed by C6–2B rat glioma cells (6). The P2Y6 receptor recently was cloned by Chang et al. (7), and this receptor subsequently was shown to be selectively activated by UDP and to be the UDP receptor natively expressed in C6–2B cells (11). The broad distribution of mRNA encoding this UDP-selective receptor suggests that it may be of widespread physiological significance (7, 8). Failure to identify this receptor in previous studies of mammalian tissues likely has been a consequence of lack of availability of potent selective agonists for uridine nucleotide receptors and the low chemical and metabolic stability of the available nucleotides. We originally reported that UDP stimulated inositol phosphate accumulation in human airway epithelial cells by low potency activation of the P2Y2 (P2U-purinergic) receptor (12, 21). However, we recently demonstrated that UDP is in fact not an agonist at the P2Y2 receptor (11), and that the previously observed effect of UDP at P2Y2 receptors can be explained by the presence of small amounts of contaminating UTP in UDP solutions and/or by conversion of UDP to UTP by cell surface nucleoside diphosphokinase.
This study illustrates that although UDP is not a P2Y2 receptor agonist, it nonetheless is a potent agonist at the human airway epithelial cell surface. The effects of UDP could not be attributed to contamination with UTP. Solutions of UDP were preincubated with hexokinase and glucose to eliminate any traces of UTP, and hexokinase and glucose were included in studies of epithelial cells to prevent metabolic conversion of UDP to UTP by cell surface nucleoside diphosphokinases and endogenously released ATP. HPLC analysis of UDP solutions before and after incubation with cells indicated the absence of UTP (data not shown). The almost equal potency of UDP and UTP on the airway cells notably contrasts with the low apparent activity of UDP at P2Y2 receptors (11). The asymmetry of the effect of UDP on polarized epithelium, with a preferred mucosal versus serosal activity, also contrasts with the predominant serosal effect of UTP (and ATP) in airway cells. Because UDP does not activate the epithelial cell P2Y2 receptor, the simplest explanation for the stimulatory effect of this diphosphate is the existence of a novel airway receptor that selectively recognizes UDP. The likely candidate for the effect of UDP in the airways is the P2Y6 receptor,* although the contribution of this receptor remains to be proven unambiguously.
The potential implications of these results are 2-fold. First, we have demonstrated that, in the presence of the apparently more abundant P2Y2 receptor, assay conditions can be established that allow resolution of a receptor that is selectively activated by UDP. Specifically, hexokinase and glucose can be used to study effects of UDP in tissues where UDP-selective effects are otherwise masked by a prominent P2Y2 receptor. Second, the asymmetry of the effects of UDP and UTP in polarized epithelial cells suggests different regulatory functions for the receptors recognizing these two nucleotides. Activators of receptors on the serosal surface may be released at distant sites and access receptors via the bloodstream, whereas UDP, which acts exclusively on the mucosal side of airway epithelial cells, may be generated locally. Whether UDP is released per se from epithelial and/or other cells remains to be shown. However, mucosal UTP is hydrolyzed at a 2- to 3-fold faster rate than mucosal UDP, and consequently, UDP likely accumulates on the apical surface after the breakdown of UTP. Thus, released UTP could be an important source of extracellular UDP, which in turn potentially serves as a physiologically important signaling molecule in human airway tissue.
Confirmation of the presence of P2Y2 receptors on the apical surface of human airway epithelial cells raised the possibility that aerosolized nucleotides might be used therapeutically to induce Cl− secretion in individuals with cystic fibrosis or other airway diseases (22). Because the expression of receptors and patterns of ion transport are similar in nasal and bronchial epithelia (23, 24), it is likely that our data in nasal epithelia are relevant to diseased lower airways in cystic fibrosis. UTP, the most potent agonist for P2Y2 receptors, is currently undergoing clinical assessment as an agent to modify airway disease in cystic fibrosis patients (25). The results of this study indicate that a receptor that selectively recognizes UDP as a potent agonist also exists in airway tissue. The functional importance of this receptor and its molecular identity need to be established rigorously, and analogues of UDP that are potent, selective, and stable agonists of this receptor need to be identified. Our initial association of this receptor with increases in Cl− secretion suggests that UDP or receptor-selective drugs derived from this natural molecule could be of potential therapeutic value for the treatment of airway diseases.
Acknowledgments
We thank the staff of E. Larsen’s laboratory (Zoophysiological Laboratory A, August Krogh Institute, University of Copenhagen, Denmark) for the miniature perfusion chamber. This work was supported by U.S. Public Health Service Grant HL32322 and Cystic Fibrosis Foundation Grant CFFR02C.
Footnotes
Reverse transcription–PCR of total RNA obtained from primary cultures of human nasal epithelium cells using oligonucleotides that specifically anneal to the third transmembrane spanning domain (forward primer) and the third intracellular loop (reverse primer) of the human P2Y6 cDNA (10) resulted in amplification of a 533-bp band as predicted for P2Y6 cDNA (E.R.L., unpublished observation).
References
- 1.Burnstock G, Kennedy C. Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 2.Dubyak G R, El-Moatassim C. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 3.Harden T K, Boyer J L, Nicholas R A. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- 4.Harden T K, Nicholas R A, Schachter J B, Lazarowski E R, Boyer J L. In: The P2-Purinergic Receptors. Weisman G A, Turner J, Fedan J, editors. Clifton, NJ: Humana; 1997. in press. [Google Scholar]
- 5.Seifert R, Schultz G. Trends Pharmacol Sci. 1989;10:365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- 6.Lazarowski E R, Harden T K. J Biol Chem. 1994;269:11830–11836. [PubMed] [Google Scholar]
- 7.Chang K, Hanaoka K, Kumada M, Takuwa Y. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- 8.Communi D, Pirotton S, Parmentier M, Boeynaems J M. J Biol Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T, Erb L, Weisman G A, Marchese A, Heng H H Q, Garrard R C, George S R, Turner J T, O’Dowd B F. J Biol Chem. 1995;270:30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- 10.Communi D, Parmentier M, Boeynaems J-M. Biochem Biophys Res Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- 11.Nicholas R A, Watt W C, Lazarowski E R, Li Q, Harden T K. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- 12.Lazarowski E R, Watt W C, Stutts M J, Boucher R C, Harden T K. Br J Pharmacol. 1995;116:1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erb L, Garrard R, Wang Y, Quinn T, Turner J T, Weisman G A. J Biol Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- 14.Wu R, Yankaskas J R, Cheng E, Knowles M R, Boucher R C. Am Rev Respir Dis. 1985;132:311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- 15.Paradiso A M, Mason S J, Lazarowski E R, Boucher R C. Nature (London) 1995;377:643–646. doi: 10.1038/377643a0. [DOI] [PubMed] [Google Scholar]
- 16.Nakahata N, Harden T K. Biochem J. 1987;241:337–344. doi: 10.1042/bj2410337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen E H, Novak I, Pedersen P S. J Physiol (London) 1990;424:109–131. doi: 10.1113/jphysiol.1990.sp018058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarowski E R, Watt W C, Stutts M J, Brown H A, Boucher R C, Harden T K. Br J Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnard E A. Methods Enzymol. 1975;42:6–20. doi: 10.1016/0076-6879(75)42085-7. [DOI] [PubMed] [Google Scholar]
- 20.Mason S J, Paradiso A M, Boucher R C. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown H A, Lazarowski E R, Boucher R C, Harden T K. Mol Pharmacol. 1991;40:648–655. [PubMed] [Google Scholar]
- 22.Knowles M R, Clarke L L, Boucher R C. N Engl J Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- 23.Boucher R C. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 24.Boucher R C. Am J Respir Crit Care Med. 1994;150:581–593. doi: 10.1164/ajrccm.150.2.8049852. [DOI] [PubMed] [Google Scholar]
- 25.Knowles M R, Olivier K N, Bennett W, Hohneker K, Geary C, Davis C W, Boucher R C. Pediatr Pulmonol Suppl. 1994;10:99. (abstr.). [Google Scholar]