Abstract
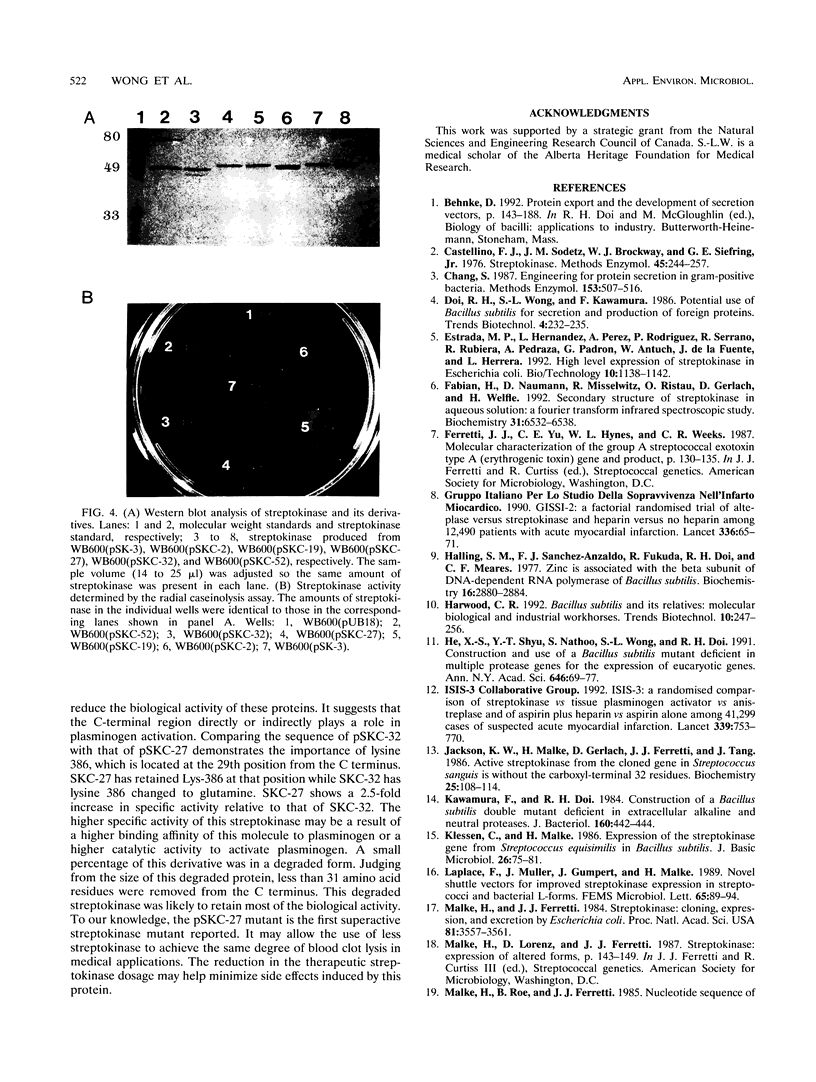
Streptokinase is one of the major blood-clot-dissolving agents used in many medical treatments. With the cloned streptokinase gene (skc) available, production of the secreted streptokinase from various Bacillus subtilis strains was studied. The use of the six-extracellular-protease-deficient strain, WB600, greatly improved the production yield of the secreted streptokinase. A modified skc which has the original skc promoter and signal sequence replaced with the B. subtilis levansucrase promoter and signal sequence was also constructed. B. subtilis carrying either the wild-type or the modified skc produces streptokinase at a comparable level. Even with WB600 as the expression host, a C-terminally-processed streptokinase was also observed. Through region-specific combinatorial mutagenesis around the C-terminal processing sites, streptokinase derivatives resistant to C-terminal degradation were engineered. One of the derivatives showed a 2.5-fold increase in specific activity and would potentially be a better thrombolytic agent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke D. Protein export and the development of secretion vectors. Biotechnology. 1992;22:143–188. [PubMed] [Google Scholar]
- Castellino F. J., Sodetz J. M., Brockway W. J., Siefring G. E., Jr Streptokinase. Methods Enzymol. 1976;45:244–257. doi: 10.1016/s0076-6879(76)45024-3. [DOI] [PubMed] [Google Scholar]
- Chang S. Engineering for protein secretion in gram-positive bacteria. Methods Enzymol. 1987;153:507–516. doi: 10.1016/0076-6879(87)53075-0. [DOI] [PubMed] [Google Scholar]
- Estrada M. P., Hernández L., Pérez A., Rodríguez P., Serrano R., Rubiera R., Pedraza A., Padrón G., Antuch W., de la Fuente J. High level expression of streptokinase in Escherichia coli. Biotechnology (N Y) 1992 Oct;10(10):1138–1142. doi: 10.1038/nbt1092-1138. [DOI] [PubMed] [Google Scholar]
- Fabian H., Naumann D., Misselwitz R., Ristau O., Gerlach D., Welfle H. Secondary structure of streptokinase in aqueous solution: a Fourier transform infrared spectroscopic study. Biochemistry. 1992 Jul 21;31(28):6532–6538. doi: 10.1021/bi00143a024. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Sanchez-Anzaldo F. J., Fukuda R., Doi R. H., Meares C. F. Zinc is associated with the beta subunit of DNA-dependent RNA polymerase of Bacillus subtilis. Biochemistry. 1977 Jun 28;16(13):2880–2884. doi: 10.1021/bi00632a012. [DOI] [PubMed] [Google Scholar]
- Harwood C. R. Bacillus subtilis and its relatives: molecular biological and industrial workhorses. Trends Biotechnol. 1992 Jul;10(7):247–256. doi: 10.1016/0167-7799(92)90233-l. [DOI] [PubMed] [Google Scholar]
- He X. S., Shyu Y. T., Nathoo S., Wong S. L., Doi R. H. Construction and use of a Bacillus subtilis mutant deficient in multiple protease genes for the expression of eukaryotic genes. Ann N Y Acad Sci. 1991 Dec 27;646:69–77. doi: 10.1111/j.1749-6632.1991.tb18565.x. [DOI] [PubMed] [Google Scholar]
- Jackson K. W., Malke H., Gerlach D., Ferretti J. J., Tang J. Active streptokinase from the cloned gene in Streptococcus sanguis is without the carboxyl-terminal 32 residues. Biochemistry. 1986 Jan 14;25(1):108–114. doi: 10.1021/bi00349a016. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessen C., Malke H. Expression of the streptokinase gene from Streptococcus equisimilis in Bacillus subtilis. J Basic Microbiol. 1986;26(2):75–81. doi: 10.1002/jobm.3620260203. [DOI] [PubMed] [Google Scholar]
- Laplace F., Müller J., Gumpert J., Malke H. Novel shuttle vectors for improved streptokinase expression in streptococci and bacterial L-forms. FEMS Microbiol Lett. 1989 Nov;53(1-2):89–94. doi: 10.1016/0378-1097(89)90371-6. [DOI] [PubMed] [Google Scholar]
- Malke H., Ferretti J. J. Streptokinase: cloning, expression, and excretion by Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3557–3561. doi: 10.1073/pnas.81.11.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H., Roe B., Ferretti J. J. Nucleotide sequence of the streptokinase gene from Streptococcus equisimilis H46A. Gene. 1985;34(2-3):357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nagarajan V. System for secretion of heterologous proteins in Bacillus subtilis. Methods Enzymol. 1990;185:214–223. doi: 10.1016/0076-6879(90)85021-f. [DOI] [PubMed] [Google Scholar]
- Park S. K., Lee B. R., Byun S. M. The leader sequence of streptokinase is responsible for its post-translational carboxyl-terminal cleavage. Biochem Biophys Res Commun. 1991 Jan 15;174(1):282–286. doi: 10.1016/0006-291x(91)90517-b. [DOI] [PubMed] [Google Scholar]
- Radek J. T., Castellino F. J. Conformational properties of streptokinase. J Biol Chem. 1989 Jun 15;264(17):9915–9922. [PubMed] [Google Scholar]
- Reidhaar-Olson J. F., Sauer R. T. Combinatorial cassette mutagenesis as a probe of the informational content of protein sequences. Science. 1988 Jul 1;241(4861):53–57. doi: 10.1126/science.3388019. [DOI] [PubMed] [Google Scholar]
- Saksela O. Radial caseinolysis in agarose: a simple method for detection of plasminogen activator in the presence of inhibitory substances and serum. Anal Biochem. 1981 Mar 1;111(2):276–282. doi: 10.1016/0003-2697(81)90564-9. [DOI] [PubMed] [Google Scholar]
- Sloma A., Rufo G. A., Jr, Theriault K. A., Dwyer M., Wilson S. W., Pero J. Cloning and characterization of the gene for an additional extracellular serine protease of Bacillus subtilis. J Bacteriol. 1991 Nov;173(21):6889–6895. doi: 10.1128/jb.173.21.6889-6895.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuten A. J., Broadhurst R. W., Smith R. A., Dobson C. M. Characterization of structural and folding properties of streptokinase by n.m.r. spectroscopy. Biochem J. 1993 Mar 1;290(Pt 2):313–319. doi: 10.1042/bj2900313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L., Wu X. C., Wong S. L. Cloning and expression of a novel protease gene encoding an extracellular neutral protease from Bacillus subtilis. J Bacteriol. 1991 Oct;173(20):6364–6372. doi: 10.1128/jb.173.20.6364-6372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L. Development of an inducible and enhancible expression and secretion system in Bacillus subtilis. Gene. 1989 Nov 30;83(2):215–223. doi: 10.1016/0378-1119(89)90107-8. [DOI] [PubMed] [Google Scholar]
- Wong S. L., Wang L. F., Doi R. H. Cloning and nucleotide sequence of senN, a novel 'Bacillus natto' (B. subtilis) gene that regulates expression of extracellular protein genes. J Gen Microbiol. 1988 Dec;134(12):3269–3276. doi: 10.1099/00221287-134-12-3269. [DOI] [PubMed] [Google Scholar]
- Wu X. C., Lee W., Tran L., Wong S. L. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol. 1991 Aug;173(16):4952–4958. doi: 10.1128/jb.173.16.4952-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. C., Nathoo S., Pang A. S., Carne T., Wong S. L. Cloning, genetic organization, and characterization of a structural gene encoding bacillopeptidase F from Bacillus subtilis. J Biol Chem. 1990 Apr 25;265(12):6845–6850. [PubMed] [Google Scholar]