Abstract
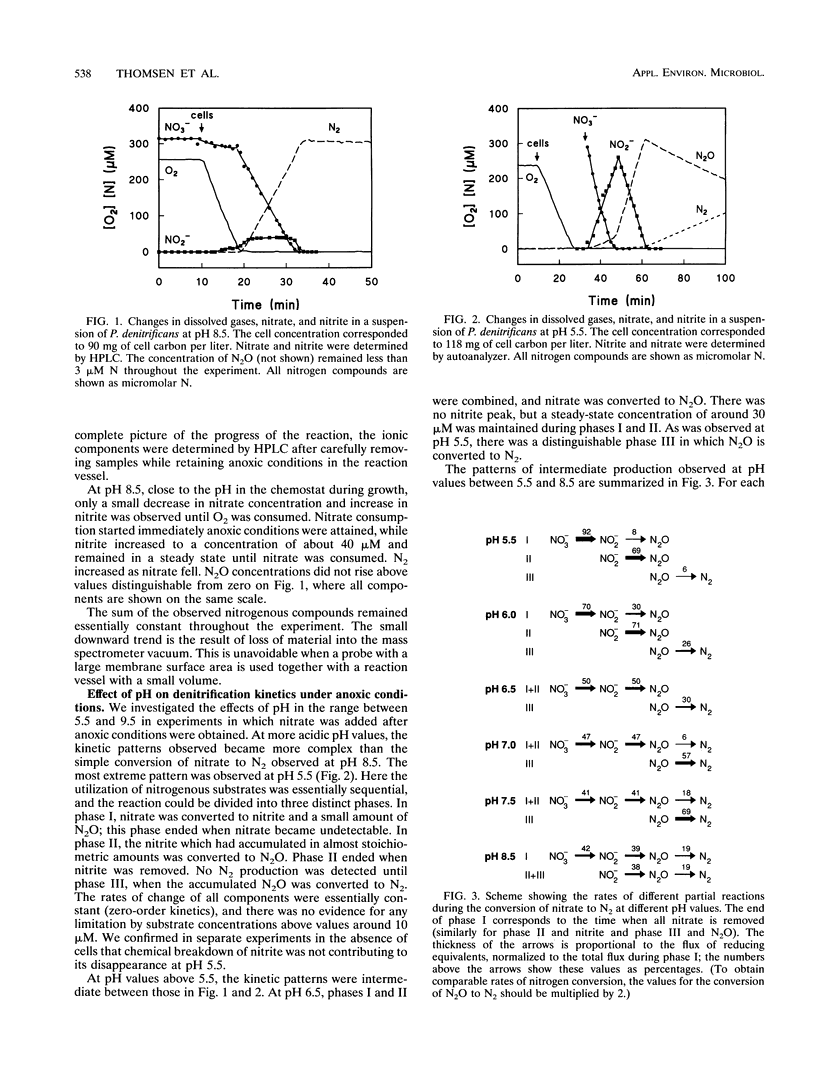
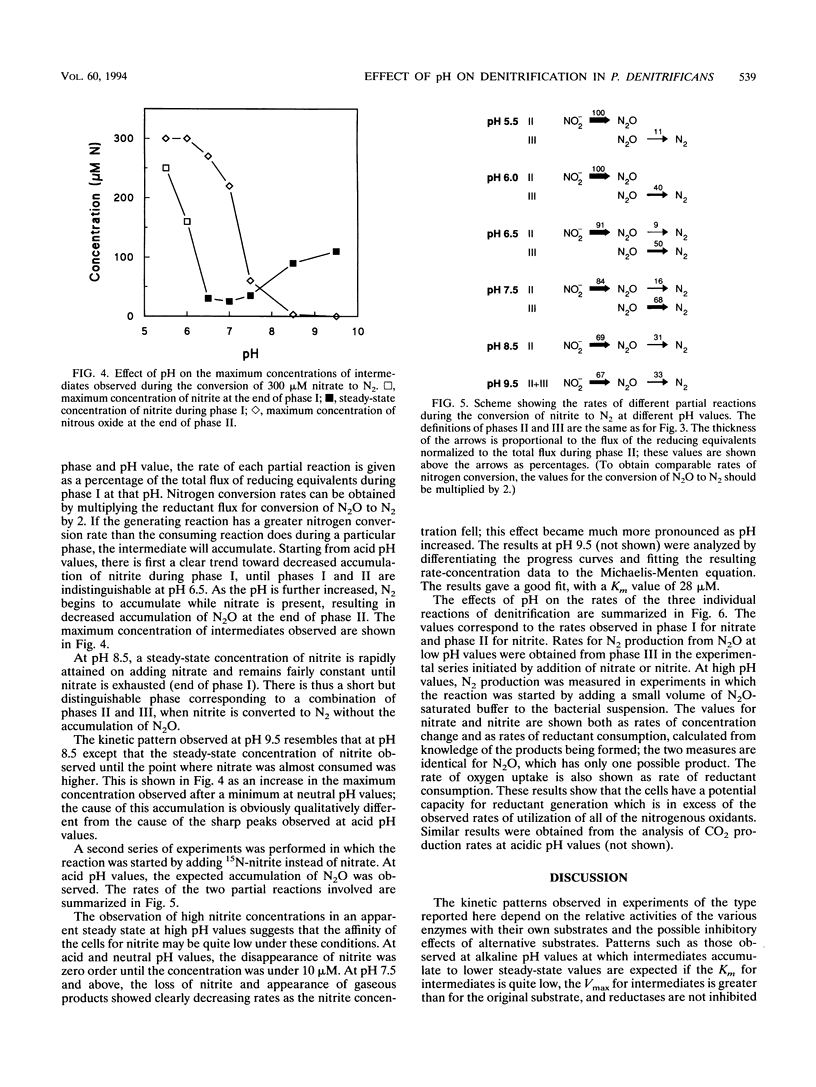
We have used a quadrupole mass spectrometer with a gas-permeable membrane inlet for continuous measurements of the production of N2O and N2 from nitrate or nitrite by cell suspensions of Paracoccus denitrificans. The use of nitrate and nitrite labeled with 15N was shown to simplify the interpretation of the results when these gases were measured. This approach was used to study the effect of pH on the production of denitrification intermediates from nitrate and nitrite under anoxic conditions. The kinetic patterns observed were quite different at acidic and alkaline pH values. At pH 5.5, first nitrate was converted to nitrite, then nitrite was converted to N2O, and finally N2O was converted to N2. At pH 8.5, nitrate was converted directly to N2, and the intermediates accumulated to only low steady-state concentrations. The sequential usage of nitrate, nitrite, and nitrous oxide observed at pH 5.5 was simulated by using a kinetic model of a branched electron transport chain in which alternative terminal reductases compete for a common reductant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betlach M. R., Tiedje J. M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981 Dec;42(6):1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. T., Lynch F. J., DeNittis A. S., Steinberg A. B., Lee H. Y., Nagele R. G. Neural tube formation in the mouse: a morphometric and computerized three-dimensional reconstruction study of the relationship between apical constriction of neuroepithelial cells and the shape of the neuroepithelium. Anat Embryol (Berl) 1990;181(1):49–58. doi: 10.1007/BF00189727. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Ingraham J. L. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl Environ Microbiol. 1983 Apr;45(4):1247–1253. doi: 10.1128/aem.45.4.1247-1253.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Lloyd D., Boddy L. The effect of oxygen on denitrification in Paracoccus denitrificans and Pseudomonas aeruginosa. J Gen Microbiol. 1989 Sep;135(9):2445–2451. doi: 10.1099/00221287-135-9-2445. [DOI] [PubMed] [Google Scholar]
- Degn H., Cox R. P., Lloyd D. Continuous measurement of dissolved gases in biochemical systems with the quadrupole mass spectrometer. Methods Biochem Anal. 1985;31:165–194. doi: 10.1002/9780470110522.ch3. [DOI] [PubMed] [Google Scholar]
- Jensen K. M., Cox R. P. Effects of sulfide and low redox potential on the inhibition of nitrous oxide reduction by acetylene in Pseudomonas nautica. FEMS Microbiol Lett. 1992 Sep 1;75(1):13–17. doi: 10.1016/0378-1097(92)90449-x. [DOI] [PubMed] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982 Mar;46(1):43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera I., Dadák V., Dobrý R. The distribution of redox equivalents in the anaerobic respiratory chain of Paracoccus denitrificans. Eur J Biochem. 1983 Feb 1;130(2):359–364. doi: 10.1111/j.1432-1033.1983.tb07161.x. [DOI] [PubMed] [Google Scholar]
- Kucera I., Lampardová L., Dadák V. Control of respiration rate in non-growing cells of Paracoccus denitrificans. Biochem J. 1987 Sep 15;246(3):779–782. doi: 10.1042/bj2460779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H. Metabolic regulation including anaerobic metabolism in Paracoccus denitrificans. J Bioenerg Biomembr. 1991 Apr;23(2):163–185. doi: 10.1007/BF00762216. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Dhesi R., Martinkus K. J., Robinson M. K., Rea T. M. Isolation of Paracoccus denitrificans cytochrome cd1: comparative kinetics with other nitrite reductases. Arch Biochem Biophys. 1982 Apr 15;215(1):47–58. doi: 10.1016/0003-9861(82)90277-6. [DOI] [PubMed] [Google Scholar]



