Abstract
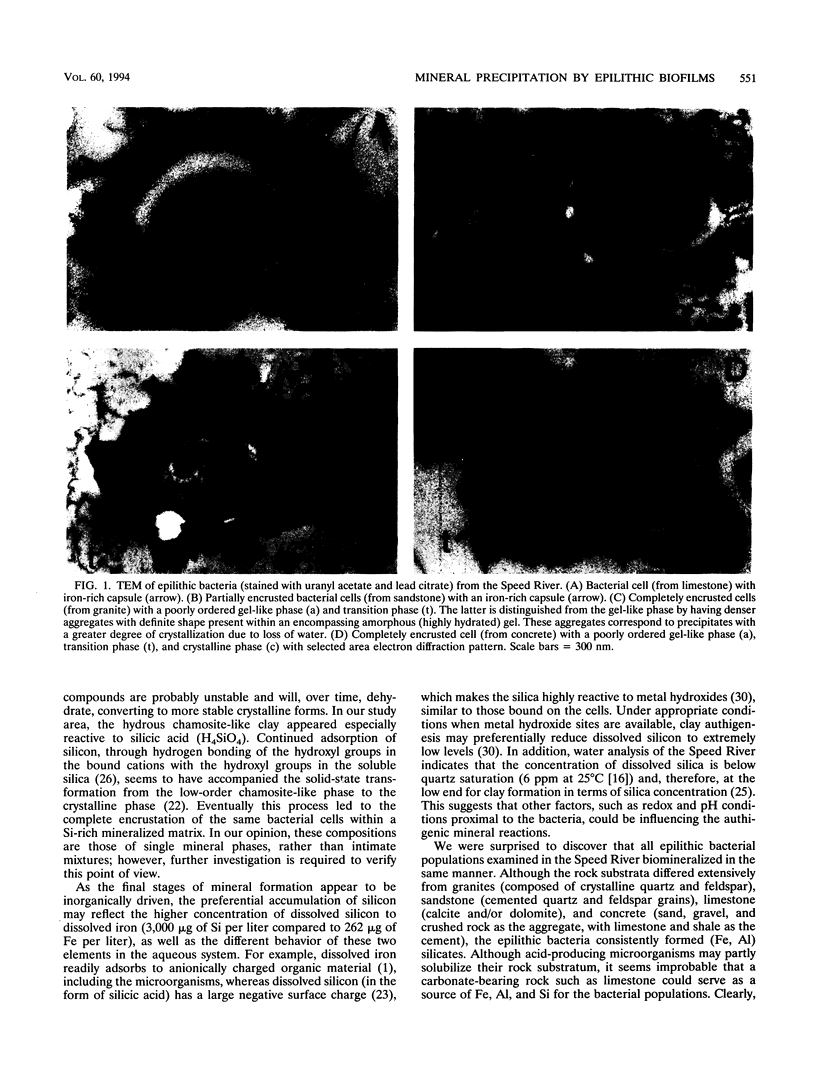
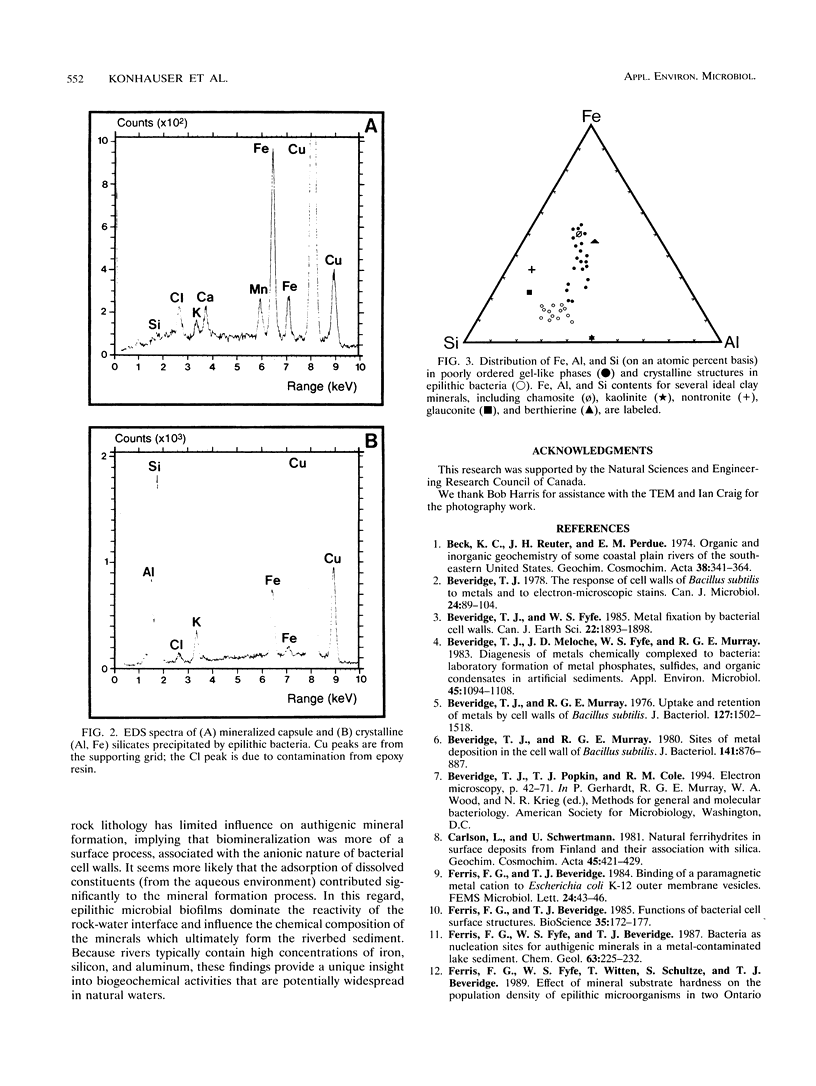
Epilithic microbial communities, ubiquitously found in biofilms on submerged granite, limestone, and sandstone, as well as on the concrete support pillars of bridges, were examined in the Speed River, Ontario, Canada. Transmission electron microscopy showed that attached bacteria (on all substrata) were highly mineralized, ranging from Fe-rich capsular material to fine-grained (<1 μm) authigenic (primary) mineral precipitates. The authigenic grains exhibited a wide range of morphologies, from amorphous gel-like phases to crystalline structures. Energy-dispersive X-ray spectroscopy indicated that the most abundant mineral associated with epilithic bacteria was a complex (Fe, Al) silicate of variable composition. The gel-like phases were similar in composition to a chamositic clay, whereas the crystalline structures were more siliceous and had compositions between those of glauconite and kaolinite. The consistent formation of (Fe, Al) silicates by all bacterial populations, regardless of substratum lithology, implies that biomineralization was a surface process associated with the anionic nature of the cell wall. The adsorption of dissolved constituents from the aqueous environment contributed significantly to the mineral formation process. In this regard, it appears that epilithic microbial biofilms dominate the reactivity of the rock-water interface and may determine the type of minerals formed, which will ultimately become part of the riverbed sediment. Because rivers typically contain high concentrations of dissolved iron, silicon, and aluminum, these findings provide a unique insight into biogeochemical activities that are potentially widespread in natural waters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Meloche J. D., Fyfe W. S., Murray R. G. Diagenesis of metals chemically complexed to bacteria: laboratory formation of metal phosphates, sulfides, and organic condensates in artificial sediments. Appl Environ Microbiol. 1983 Mar;45(3):1094–1108. doi: 10.1128/aem.45.3.1094-1108.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976 Sep;127(3):1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. The response of cell walls of Bacillus subtilis to metals and to electron-microscopic stains. Can J Microbiol. 1978 Feb;24(2):89–104. doi: 10.1139/m78-018. [DOI] [PubMed] [Google Scholar]
- Ferris F. G., Schultze S., Witten T. C., Fyfe W. S., Beveridge T. J. Metal Interactions with Microbial Biofilms in Acidic and Neutral pH Environments. Appl Environ Microbiol. 1989 May;55(5):1249–1257. doi: 10.1128/aem.55.5.1249-1257.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen M. D., Wolf D. C., Ferris F. G., Beveridge T. J., Flemming C. A., Bailey G. W. Bacterial sorption of heavy metals. Appl Environ Microbiol. 1989 Dec;55(12):3143–3149. doi: 10.1128/aem.55.12.3143-3149.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp R. J., Pfaender F. K. Effects of surface area and flow rate on marine bacterial growth in activated carbon columns. Appl Environ Microbiol. 1982 Aug;44(2):471–477. doi: 10.1128/aem.44.2.471-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]