Abstract
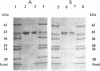
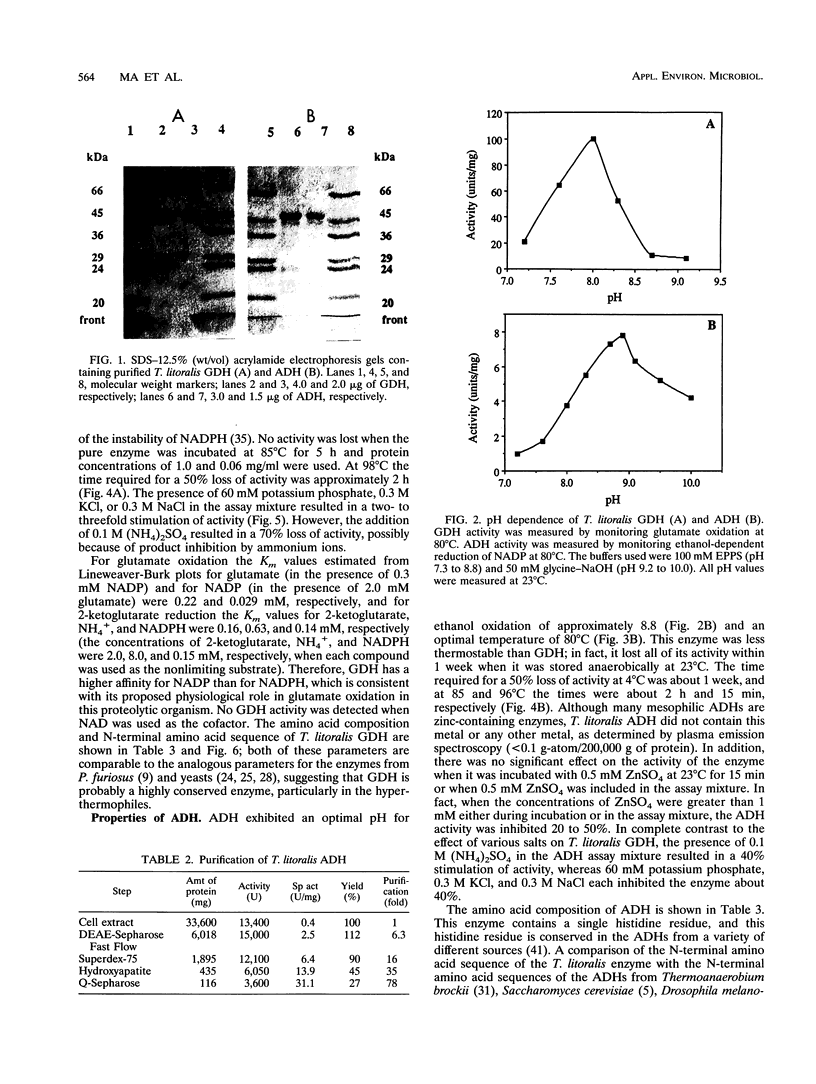
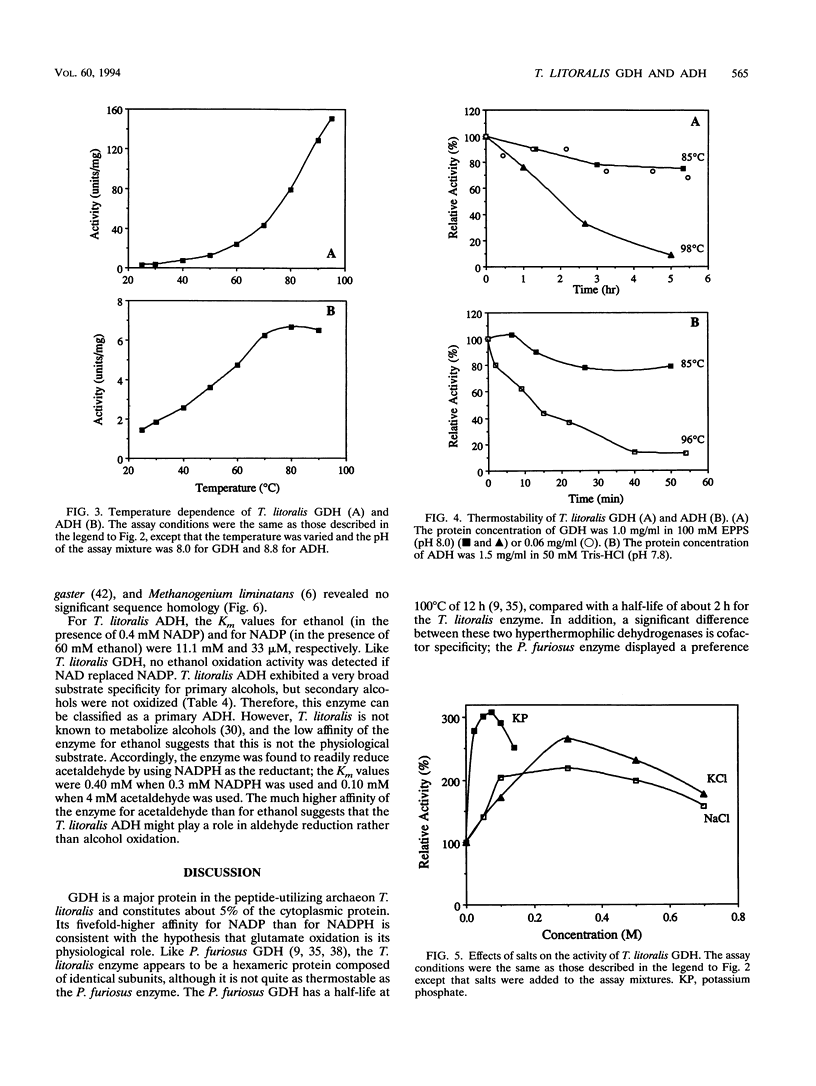
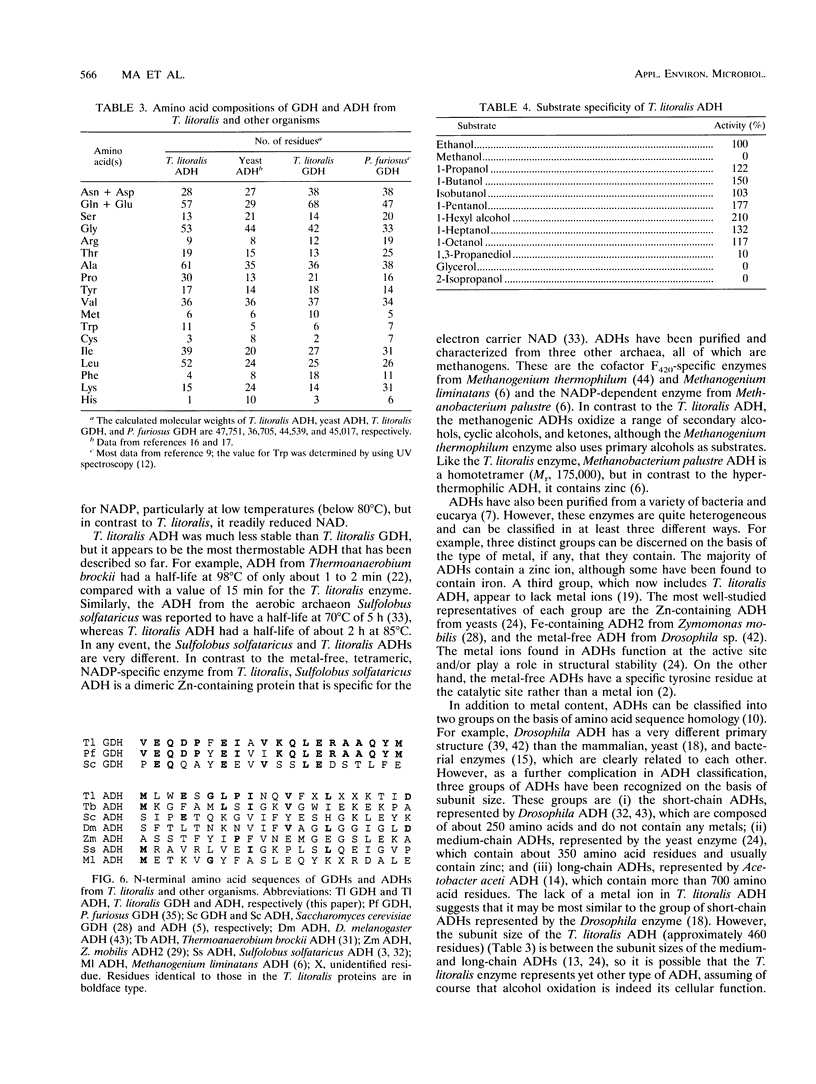
Thermococcus litoralis is a strictly anaerobic archaeon that grows at temperatures up to 98 degrees C by fermenting peptides. Little is known about the primary metabolic pathways of this organism and, in particular, the role of enzymes that are dependent on thermolabile nicotinamide nucleotides. In this paper we show that the cytoplasmic fraction of cell extracts contained NADP-specific glutamate dehydrogenase (GDH) and NADP-specific alcohol dehydrogenase (ADH) activities, neither of which utilized NAD as a cofactor. The GDH is composed of identical subunits having an M(r) of 45,000 and had an optimal pH and optimal temperature for glutamate oxidation of 8.0 and > 95 degrees C, respectively. Potassium phosphate (60 mM), KCl (300 mM), and NaCl (300 mM) each stimulated the rate of glutamate oxidation activity between two- and threefold. For glutamate oxidation the apparent Km values at 80 degrees C for glutamate and NADP were 0.22 and 0.029 mM, respectively, and for 2-ketoglutarate reduction the apparent Km values for 2-ketoglutarate, NADPH, and NH4+ were 0.16, 0.14, and 0.63 mM, respectively. This enzyme is the first NADP-specific GDH purified form a hyperthermophilic organism. T. litoralis ADH is a tetrameric protein composed of identical subunits having an M(r) of 48,000; the optimal pH and optimal temperature for ethanol oxidation were 8.8 and 80 degrees C, respectively. In contrast to GDH activity, potassium phosphate (60 mM), KCl (0.1 M), and NaCl (0.3 M) inhibited ADH activity, whereas (NH4)2SO4 (0.1 M) had a slight stimulating effect. This enzyme exhibited broad substrate specificity for primary alcohols, but secondary alcohols were not oxidized.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W. Enzymes and proteins from organisms that grow near and above 100 degrees C. Annu Rev Microbiol. 1993;47:627–658. doi: 10.1146/annurev.mi.47.100193.003211. [DOI] [PubMed] [Google Scholar]
- Albalat R., González-Duarte, Atrian S. Protein engineering of Drosophila alcohol dehydrogenase. The hydroxyl group of Tyr152 is involved in the active site of the enzyme. FEBS Lett. 1992 Aug 24;308(3):235–239. doi: 10.1016/0014-5793(92)81282-q. [DOI] [PubMed] [Google Scholar]
- Ammendola S., Raia C. A., Caruso C., Camardella L., D'Auria S., De Rosa M., Rossi M. Thermostable NAD(+)-dependent alcohol dehydrogenase from Sulfolobus solfataricus: gene and protein sequence determination and relationship to other alcohol dehydrogenases. Biochemistry. 1992 Dec 15;31(49):12514–12523. doi: 10.1021/bi00164a031. [DOI] [PubMed] [Google Scholar]
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Bleicher K., Winter J. Purification and properties of F420- and NADP(+)-dependent alcohol dehydrogenases of Methanogenium liminatans and Methanobacterium palustre, specific for secondary alcohols. Eur J Biochem. 1991 Aug 15;200(1):43–51. doi: 10.1111/j.1432-1033.1991.tb21046.x. [DOI] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Consalvi V., Chiaraluce R., Politi L., Vaccaro R., De Rosa M., Scandurra R. Extremely thermostable glutamate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus furiosus. Eur J Biochem. 1991 Dec 18;202(3):1189–1196. doi: 10.1111/j.1432-1033.1991.tb16489.x. [DOI] [PubMed] [Google Scholar]
- Danielsson O., Jörnvall H. "Enzymogenesis": classical liver alcohol dehydrogenase origin from the glutathione-dependent formaldehyde dehydrogenase line. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9247–9251. doi: 10.1073/pnas.89.19.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Inoue T., Sunagawa M., Mori A., Imai C., Fukuda M., Takagi M., Yano K. Cloning and sequencing of the gene encoding the 72-kilodalton dehydrogenase subunit of alcohol dehydrogenase from Acetobacter aceti. J Bacteriol. 1989 Jun;171(6):3115–3122. doi: 10.1128/jb.171.6.3115-3122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck R., Woenckhaus C., Harris J. J., Runswick M. J. Identification of the amino acid residue modified in Bacillus stearothermophilus alcohol dehydrogenase by the NAD+ analogue 4-(3-bromoacetylpyridinio)butyldiphosphoadenosine. Eur J Biochem. 1979 Jan 2;93(1):57–64. doi: 10.1111/j.1432-1033.1979.tb12794.x. [DOI] [PubMed] [Google Scholar]
- Jeffery J., Cummins L., Carlquist M., Jörnvall H. Properties of sorbitol dehydrogenase and characterization of a reactive cysteine residue reveal unexpected similarities to alcohol dehydrogenases. Eur J Biochem. 1981 Nov;120(2):229–234. doi: 10.1111/j.1432-1033.1981.tb05693.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Eklund H., Brändén C. I. Subunit conformation of yeast alcohol dehydrogenase. J Biol Chem. 1978 Dec 10;253(23):8414–8419. [PubMed] [Google Scholar]
- Jörnvall H., Persson B., Jeffery J. Characteristics of alcohol/polyol dehydrogenases. The zinc-containing long-chain alcohol dehydrogenases. Eur J Biochem. 1987 Sep 1;167(2):195–201. doi: 10.1111/j.1432-1033.1987.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. The primary structure of yeast alcohol dehydrogenase. Eur J Biochem. 1977 Feb;72(3):425–442. doi: 10.1111/j.1432-1033.1977.tb11267.x. [DOI] [PubMed] [Google Scholar]
- Klump H., Di Ruggiero J., Kessel M., Park J. B., Adams M. W., Robb F. T. Glutamate dehydrogenase from the hyperthermophile Pyrococcus furiosus. Thermal denaturation and activation. J Biol Chem. 1992 Nov 5;267(31):22681–22685. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magonet E., Hayen P., Delforge D., Delaive E., Remacle J. Importance of the structural zinc atom for the stability of yeast alcohol dehydrogenase. Biochem J. 1992 Oct 15;287(Pt 2):361–365. doi: 10.1042/bj2870361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye W. S., Amuro N., Rao J. K., Zalkin H. Nucleotide sequence of yeast GDH1 encoding nicotinamide adenine dinucleotide phosphate-dependent glutamate dehydrogenase. J Biol Chem. 1985 Jul 15;260(14):8502–8508. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon, Thermococcus litoralis. A role for tungsten in peptide catabolism. J Biol Chem. 1993 Jun 25;268(18):13592–13600. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991 Aug 5;266(22):14208–14216. [PubMed] [Google Scholar]
- Nagasu T., Hall B. D. Nucleotide sequence of the GDH gene coding for the NADP-specific glutamate dehydrogenase of Saccharomyces cerevisiae. Gene. 1985;37(1-3):247–253. doi: 10.1016/0378-1119(85)90279-3. [DOI] [PubMed] [Google Scholar]
- Neale A. D., Scopes R. K., Kelly J. M., Wettenhall R. E. The two alcohol dehydrogenases of Zymomonas mobilis. Purification by differential dye ligand chromatography, molecular characterisation and physiological roles. Eur J Biochem. 1986 Jan 2;154(1):119–124. doi: 10.1111/j.1432-1033.1986.tb09366.x. [DOI] [PubMed] [Google Scholar]
- Peretz M., Burstein Y. Amino acid sequence of alcohol dehydrogenase from the thermophilic bacterium Thermoanaerobium brockii. Biochemistry. 1989 Aug 8;28(16):6549–6555. doi: 10.1021/bi00442a004. [DOI] [PubMed] [Google Scholar]
- Persson B., Krook M., Jörnvall H. Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem. 1991 Sep 1;200(2):537–543. doi: 10.1111/j.1432-1033.1991.tb16215.x. [DOI] [PubMed] [Google Scholar]
- Rella R., Raia C. A., Pensa M., Pisani F. M., Gambacorta A., De Rosa M., Rossi M. A novel archaebacterial NAD+-dependent alcohol dehydrogenase. Purification and properties. Eur J Biochem. 1987 Sep 15;167(3):475–479. doi: 10.1111/j.1432-1033.1987.tb13361.x. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Robb F. T., Park J. B., Adams M. W. Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium, Pyrococcus furiosus. Biochim Biophys Acta. 1992 Apr 17;1120(3):267–272. doi: 10.1016/0167-4838(92)90247-b. [DOI] [PubMed] [Google Scholar]
- Schicho R. N., Ma K., Adams M. W., Kelly R. M. Bioenergetics of sulfur reduction in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1993 Mar;175(6):1823–1830. doi: 10.1128/jb.175.6.1823-1830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkinger M. F., Redl B., Stöffler G. Purification and properties of an extreme thermostable glutamate dehydrogenase from the archaebacterium Sulfolobus solfataricus. Biochim Biophys Acta. 1991 Jan 23;1073(1):142–148. doi: 10.1016/0304-4165(91)90194-l. [DOI] [PubMed] [Google Scholar]
- Schwartz M. F., Jörnvall H. Structural analyses of mutant and wild-type alcohol dehydrogenases from drosophila melanogaster. Eur J Biochem. 1976 Sep;68(1):159–168. doi: 10.1111/j.1432-1033.1976.tb10774.x. [DOI] [PubMed] [Google Scholar]
- Sun H. W., Plapp B. V. Progressive sequence alignment and molecular evolution of the Zn-containing alcohol dehydrogenase family. J Mol Evol. 1992 Jun;34(6):522–535. doi: 10.1007/BF00160465. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R. The complete amino acid sequence of three alcohol dehydrogenase alleloenzymes (AdhN-11, AdhS and AdhUF) from the fruitfly Drosophila melanogaster. Biochem J. 1980 Jun 1;187(3):875–883. doi: 10.1042/bj1870875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya A., Juan E., Egestad B., Jörnvall H. The primary structure of alcohol dehydrogenase from Drosophila lebanonensis. Extensive variation within insect 'short-chain' alcohol dehydrogenase lacking zinc. Eur J Biochem. 1989 Mar 1;180(1):191–197. doi: 10.1111/j.1432-1033.1989.tb14632.x. [DOI] [PubMed] [Google Scholar]