Abstract
The widespread use and relative persistence of s-triazine compounds such as atrazine and simazine have led to increasing concern about environmental contamination by these compounds. Few microbial isolates capable of transforming substituted s-triazines have been identified. Rhodococcus corallinus NRRL B-15444 has previously been shown to possess a hydrolase activity that is responsible for the dechlorination of the triazine compounds deethylsimazine (6-chloro-N-ethyl-1,3,5-triazine-2,4-diamine) (CEAT) and deethylatrazine (6-chloro-N-isopropyl-1,3,5-triazine-2,4-diamine) (CIAT). The enzyme responsible for this activity was purified and shown to be composed of four identical subunits of 54,000 Da. Kinetic experiments revealed that the purified enzyme is also capable of deaminating the structurally related s-triazine compounds melamine (2,4,6-triamino-1,3,5-triazine) (AAAT) and CAAT (2-chloro-4,6-diamino-1,3,5-triazine), as well as the pyrimidine compounds 2,4,6-triaminopyrimidine (AAAP) and 4-chloro-2,6-diaminopyrimidine (CAAP). The triazine herbicides atrazine and simazine inhibit the hydrolytic activities of the enzyme but are not substrates. Induction experiments demonstrate that triazine hydrolytic activity is inducible and that this activity rises approximately 20-fold during induction.
Full text
PDF

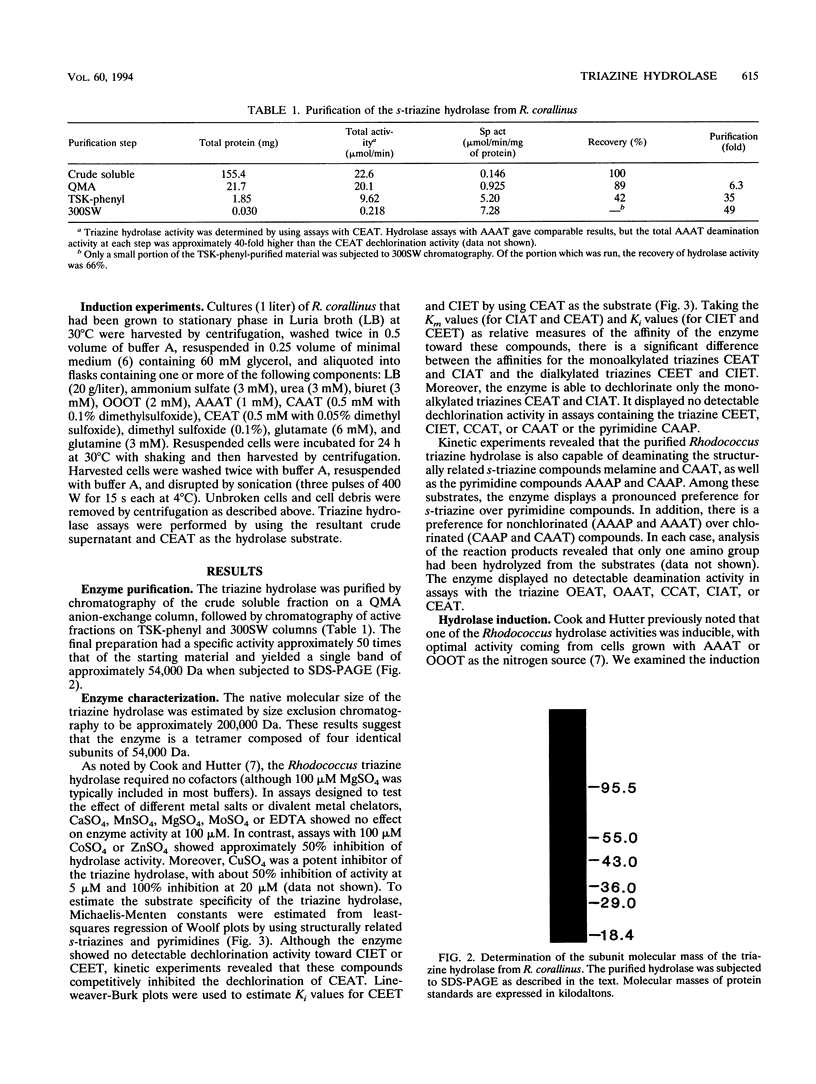
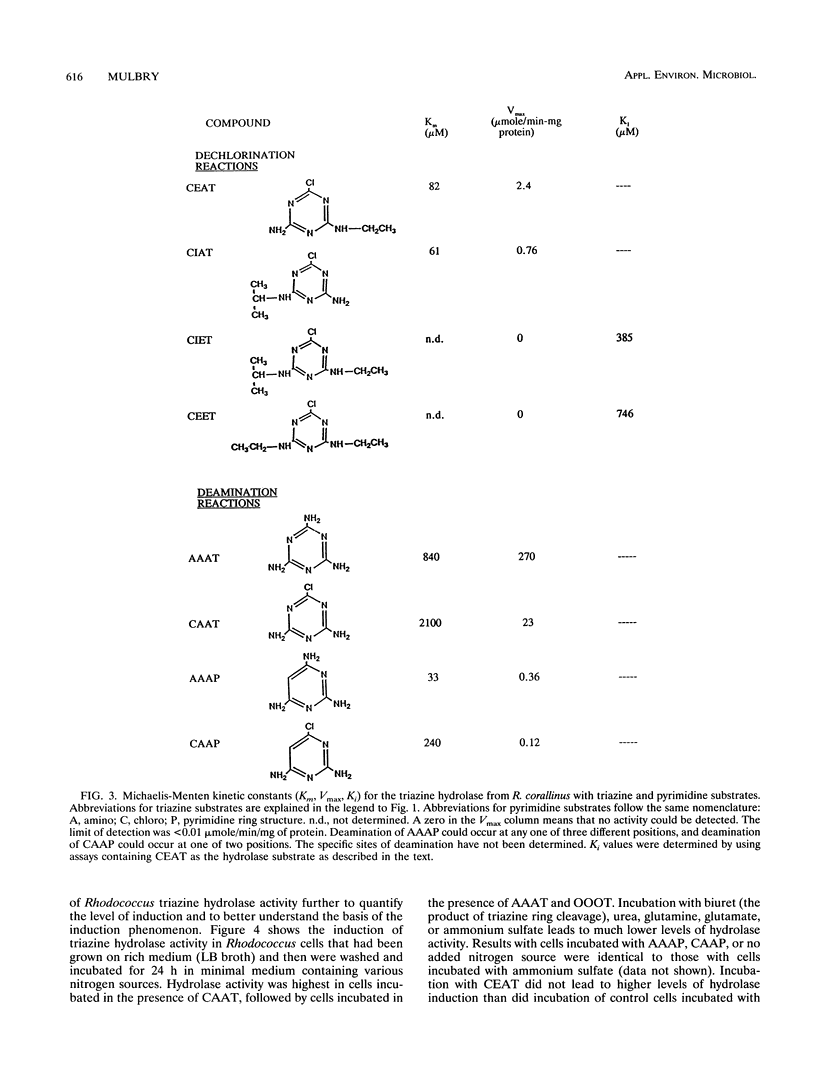


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behki R., Topp E., Dick W., Germon P. Metabolism of the herbicide atrazine by Rhodococcus strains. Appl Environ Microbiol. 1993 Jun;59(6):1955–1959. doi: 10.1128/aem.59.6.1955-1959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Eaton R. W., Karns J. S. Cloning and analysis of s-triazine catabolic genes from Pseudomonas sp. strain NRRLB-12227. J Bacteriol. 1991 Feb;173(3):1215–1222. doi: 10.1128/jb.173.3.1215-1222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Karns J. S. Cloning and comparison of the DNA encoding ammelide aminohydrolase and cyanuric acid amidohydrolase from three s-triazine-degrading bacterial strains. J Bacteriol. 1991 Feb;173(3):1363–1366. doi: 10.1128/jb.173.3.1363-1366.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandelbaum R. T., Wackett L. P., Allan D. L. Mineralization of the s-triazine ring of atrazine by stable bacterial mixed cultures. Appl Environ Microbiol. 1993 Jun;59(6):1695–1701. doi: 10.1128/aem.59.6.1695-1701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]




