Abstract
Much of the knowledge about cryptochrome function in Drosophila stems from analyzing the cryb mutant. Several features of this variant's light responsiveness imply either that CRYb retains circadian-photoreceptive capacities or that additional CRY-independent light-input routes subserve these processes. Potentially to resolve these issues, we generated cry knock-out mutants (cry0's) by gene replacement. They behaved in an anomalously rhythmic manner in constant light (LL). However, cry0 flies frequently exhibited two separate circadian components in LL, not observed in most previous cryb analyses. Temperature-dependent circadian phenotypes exhibited by cry0 flies suggest that CRY is involved in core pacemaking. Further locomotor experiments combined cry0 with an externally blinding mutation (norpAP24), which caused the most severe decrements of circadian photoreception observed so far. cryb cultures were shown previously to exhibit either aperiodic or rhythmic eclosion in separate studies. We found cry0 to eclose in a solidly periodic manner in light:dark cycles or constant darkness. Furthermore, both cry0 and cryb eclosed rhythmically in LL. These findings indicate that the novel cry0 type causes more profound defects than does the cryb mutation, implying that CRYb retains residual activity. Because some norpAP24 cry0 individuals can resynchronize to novel photic regimes, an as-yet undetermined light-input route exists in Drosophila.
CIRCADIAN clocks evolved in most organisms in part to mediate the internal “temporal order” that is associated with various biological phenomena, and, furthermore, such that an animal, plant, or microbe can anticipate everyday periodic changes of light and temperature. Light comprises the principal clock resetting cue, which keeps the process running at a 24.0-hr pace in natural conditions (Dunlap et al. 2004). Thermal cycles can also “entrain” circadian clocks; and another aspect of how these processes respond to temperature changes is to compensate such that the pacemaker operates at the same velocity in different thermal conditions (Dunlap et al. 2004).
One way by which Drosophila melanogaster's version of a cryptochrome (CRY) protein was implicated in circadian rhythms was by screening for mutations that would perturb luciferase (luc)-reported expression of a canonical clock gene called period (per). The seminal cryb mutation was thus recognized because it eliminated the normal daily cycles of luminescence mediated by a per-luc transgene in real-time monitorings of whole flies (Stanewsky et al. 1998). This mutation entails an amino-acid substitution within the fly's CRY at a site believed to be involved in binding a flavin moiety that participates in the protein's capacity to absorb blue light (Stanewsky et al. 1998; cf. Sancar 2000).
Most of the “glow cycling” emanating from cry+ (and otherwise rhythm-normal) Drosophila comes from peripheral tissues (Stanewsky et al. 1997), including several appendages projecting from the adult animal (Plautz et al. 1997; Levine et al. 2002b). The effects of the cryb mutation on such molecular rhythmicity implied that the normal gene is expressed in the periphery, but cry+ was found also to make its products in the central nervous system (Emery et al. 2000b; Klarsfeld et al. 2004). In one test of cryb's effects on whole-animal rhythm-related phenotypes, mutant individuals resynchronized their behavior in a largely normal manner to shifted light:dark (LD) cycles; “L” in the postshift regime was dim blue light (Stanewsky et al. 1998). However this mutant type required longer-than-normal times to resynchronize (Emery et al. 2000b). Much poorer photic sensitivity was observed when a no-receptor-potential-A mutation (norpAP41), which probably causes blindness at the level of all external photoreceptors, was added to a cryb genetic background. However, appreciable proportions of the norpAP41cryb double mutants were capable of re-entraining their locomotor cycles to shifted photic regimes that involved dim blue light (Stanewsky et al. 1998; see also Helfrich-Förster et al. 2001; Mealey-Ferrara et al. 2003). More severe entrainment problems were observed when cryb was combined with a glass eye mutation (gl60j), which eliminates external photoreceptive structures and a putative “extra-ocular” photoreceptor called the Hofbauer–Buchner (H–B) eyelet (Helfrich-Förster et al. 2001; Mealey-Ferrara et al. 2003). The H–B structure is located underneath the compound eye at the distal extremity of the CNS's optic lobes (Hofbauer and Buchner 1989).
Issues revolving round a circadian-photoreceptive role played by the H–B eyelet connect with chrono-genetic variants produced for or applied in the current study: Consider that the normal form of the aforementioned norpA gene is expressed not only in the compound eyes and ocelli, but also within H–B eyelet photoreceptors—along with Rhodopsin gene products that are possessed by such extra-ocular photoreceptive cells (Malpel et al. 2002). Therefore, one could predict that all photic input routes to the clock should be blinded in a norpA cryb double mutant, with the proviso that both variants are null. But Drosophila suffering the simultaneous effects of these mutations were re-entrainable (see above), and a norpA-independent function has recently been re-argued as to how the H–B structure operates within the fly's circadian system (Veleri et al. 2007). With regard to the eyelet-eliminating effect of gl60j, the enhanced degradation of rhythm-related photoreception caused by combining that mutation with cryb (see above) involved an overinterpretation of the extent to which that doubly mutant type is circadian blind (see Mealey-Ferrara et al. 2003 and further discussions of this matter within the current article). More generally, one can denigrate the glass mutation as a dull experimental tool: The gl60j mutant is likely to possess pleiotropic abnormalities beyond its lack of eyes and H–B eyelets; that this genetic variant could be brain damaged in only partly appreciated ways is implied by elements of the findings in Helfrich-Förster et al. (2001) and Klarsfeld et al. (2004).
In any case, these mutational effects indicated that the entrainment pathway that keeps Drosophila behaving in synchrony with LD cycles entails multiple input routes. That one of them includes photoreception by CRY in the head was signified by two further tests (Stanewsky et al. 1998; Emery et al. 2000a): (i) cryb individuals were found not to exhibit locomotor phase shifts after relatively brief light pulses were delivered to flies otherwise maintained in constant darkness (DD) and (ii) cryb behaved in an anomalously rhythmic manner in LL. Genetically normal Drosophila behave arrhythmically when the LL intensity is rather high but exhibit longer than normal (compared with DD) locomotor periods in dim light (Konopka et al. 1989). By comparison, cryb individuals have been reported either to display periodicities in LL that are either equivalent to the free-running behavior of wild-type Drosophila in DD (Emery et al. 2000a) or entail slightly lengthened cycle durations (Helfrich-Förster et al. 2001; Mealey-Ferrara et al. 2003). If the latter sets of results are accurate, the suggestion arises that this mutant is not completely “circadian-blind” in this kind of photic situation: cryb flies would be perceiving an ordinary level of LL as dim light.
“Deep brain” expression of the normal cry coding sequence has been deduced to connect with elements of these behavioral phenomena. Driving cry+ with a Pdf-gal4 transgene, which is expressed in the brain only within ∼10 pairs of so-called ventro-lateral neurons (LNv's) and in the posterior-most ganglion of the ventral nerve cord (Park et al. 2000), resulted in partial “rescue” of cryb-induced insensitivity to light pulses and abnormal rhythmicity in LL (Emery et al. 2000a,b). Rescue of the mutant's light responsiveness was better (Stoleru et al. 2007) when cry+ was transgenically restored to dorsal LN's, which are also among the brain cells expressing clock genes.
In adult appendages, cryb caused poor cycling of per and timeless (tim) and essentially quashed a daily cycle of odor sensitivity that normally operates in the antenna (cf. Krishnan et al. 1999); these results led to the suggestion that CRY functions as a “clock protein” in the periphery (Krishnan et al. 2001; cf. Ivanchenko et al. 2001; Collins et al. 2006). This supposition was considered further in the context of cryb's (partial) disruption of molecular rhythms in isolated peripheral-tissue specimens (Levine et al. 2002a,b). However, monitoring luciferase-reported cyclings of clock genes in cultured appendages implied that residual per and tim gene-expression rhythmicities may still be running in cryb specimens (Krishnan et al. 2001; Levine et al. 2002b). In contrast, clock function in the CNS remains robust under the influence of this mutation. For example, free-running behavioral rhythmicity was found to be unimpaired in cryb flies in DD (e.g., Stanewsky et al. 1998; Mealey-Ferrara et al. 2003). However, when CRY was caused to be “constitutively active,” longer-than-normal behavioral cycles were observed (Busza et al. 2004; Dissel et al. 2004); and when cryb was added to a genotype that causes overexpression of a “clock kinase” and shortened free-running periods, the locomotor cycle durations were pushed closer to the normal range (Stoleru et al. 2007).
The cryb mutation causes a lowering of CRY levels sufficient to reveal the participation of this factor in circadian photoreception and in peripheral timekeeping. However, residual CRY activity associated with both such processes suggests that cryb is not a loss-of-function mutant. Initially, CRY immunoreactivity was undetectable in homogenates of this mutant type (Stanewsky et al. 1998). However, that antibody is no longer available, and applications of an anti-CRY subsequently produced by Busza et al. (2004) indicated that cryb “leaks out” a low level of its encoded protein; additionally, these investigators generated a new cryptochrome mutant (crym), which also is hypomorphic. Key features of these findings prompt the hypothesis that the existing mutants retain enough functional CRY to allow for the various rhythmic attributes now being surveyed. Alternatively, cryb is a null mutant; and alternative input routes that do not use CRY would mediate light-to-clock signaling in flies suffering the effects of an extant cry mutant, including in those that carry an additional visual-system mutation (norpA or glass).
Therefore, we felt that an expansion of the allelic series for cryptochrome in D. melanogaster was warranted. We set out to target this autosomal locus by the transposon-based tactics that allow for exogenous DNA sequences to be engineered into a specific Drosophila gene (reviewed in Bi and Rong 2003; Venken and Bellen 2005). We then analyzed rhythms of behavior and of luc-reported clock-gene expression in cry-null flies and their peripheral tissues.
One more reason for creating a new cry mutant connects with whether or not CRY-depleting genetic variants affect the circadian rhythm accompanying eclosion (emergence of adults from metamorphosis). It was assumed that the compound eyes are not involved in transmitting light inputs toward the clock that underlies periodic eclosion, because D. pseudoobscura cultures dramatically depleted of retinal (required for the function of compound-eye rhodopsins) still can be synchronized by photic inputs to exhibit rhythmic eclosion with undiminished sensitivity (Zimmerman and Goldsmith 1971). In contrast, dietary depletion of retinal causes markedly subnormal sensitivity of the fly's circadian system insofar as light-induced synchronization of adult locomotion is concerned (Ohata et al. 1998). Furthermore, the most efficacious light subserving synchrony of developing Drosophila is in the blue range (Frank and Zimmerman 1969; Klemm and Ninnemann 1976), where CRY absorbs maximally (Sancar 2000). Therefore, one could readily predict that cryb cultures would eclose aperiodiocially—a result that was reported in one study (Myers et al. 2003); but dramatically different effects stemmed from another one (Mealey-Ferrara et al. 2003). We surmised that testing effects on eclosion of a novel, even more severely mutated, cry allele could resolve this conundrum.
MATERIALS AND METHODS
Construct for homologous recombination into the cry locus:
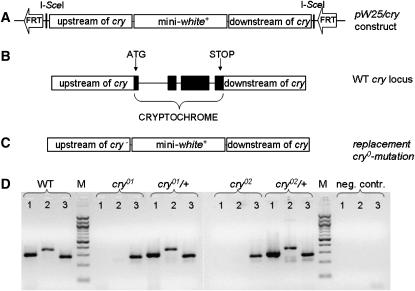
A plasmid derived from a vector pW25 (Gong and Golic 2003) was modified to contain separate pieces of third chromosomal DNA that closely flank the cry locus. This vector otherwise contains a shortened version of the white gene (intronless mini-white+, hereafter called w+) and two FRT sequences that are substrates for flipase (FLP) recombinase (see Figure 1A). The “near-cry” sequences inserted into pW25 were two genomic fragments, one containing a 5′-flanking and the other containing a 3′-flanking sequence. They were amplified by PCR from DNA extracted from Canton-S wild type (stock no. 1 at the Bloomington Drosophila Stock Center, Indiana University, Bloomington, IN), using the following primers: (1) to produce the 5′ locus-flanking fragment (consisting of 2944 bp): CRYf-Acc65I, 5′-CAGGTACCGTTTTATTTGGTTTCGTTTTAC; CRYr-SphI, 5′-CAGCATGCCGCGGTCCCCTGGTG; and (2) the 3′ fragment (2826 bp): CRY2f-BsiWI, 5′-CACGTACGCAGGCGAGGAGACAAC; CRY2r-AscI, 5′-CAGGCGCGCCATGCGAAATTTGAGTGCTGAGAA. The two amplicons were ligated into pW25 restriction sites (for the 2.9-kb fragment: Acc65I/SphI; for the 2.8-kb one: BsiWI/AscI). Thus, the pW25/cry vector carries w+ flanked by the two pieces of near-cry DNA; located outside of those 2.9- and 2.8-kb fragments are the FRTs and two I-SceI sites (Figure 1).
Figure 1.—
HR into the cry locus to knock it out. (A) Design of pW25/cry transformation vector. This plasmid carries mini-white+ marker gene surrounded by segments of third-chromosomal DNA flanking the cry gene on either side (upstream and downstream of cry coding sequence), along with two FRT sequences and two recognition sites for the I-SceI endonuclease. (B) Genomic organization of the wild-type (WT) cry+ gene with translation start and stop sites indicated. (C) Genomic organization of newly created cry locus after HR. The entire coding sequence of cry was replaced by mini-white+. (D) PCR analysis of different cry-locus forms. Genomic DNA was isolated from adult flies, homozygous or heterozygous for the third chromosome carrying the putative targeted HR event. PCR was also carried out on DNA from control WT flies and without any DNA. Flies heterozygous for a given cry0 mutation (cry01 or cry02) carried the normal allele in a third-chromosomal balancer called TM3 (see materials and methods). Three sets of primers were used for each DNA sample (see materials and methods for such sequences). Lane 1, amplification product of the second cry exon. Lane 2, amplification of a DNA fragment near the 3′ end of cry's third exon. Lane 3, amplification of Clock gene sequence, to gauge the integrity of these PCRs. Lane M, 100-bp DNA ladder (New England BioLabs, Ipswich, MA), applied to calibrate PCR product lengths.
Molecular procedures and genetic crosses for creation of transgenic lines:
The pW25/cry construct aimed at mediating HR was injected into y w embryos whose third chromosome contained the transposase-encoding Δ2-3 variant (Robertson et al. 1988). Otherwise, the procedure for producing germline P-element transformants was conventional (e.g., Rubin and Spradling 1982; Park and Lim 1995). Flies developing from the injected embryos were crossed individually to y w In(2LR)O, Cy/Bl, and also to y w In(3LR)TM3, Sb Ser/H or w In(3LR)TM3, Sb Ser/In(3LR)TM6B, Hu (as an alternative for tracking the inheritance of third chromosomes). Backcrossing non-w progeny (each of which carried a given second or third chromosome dominant marker) to the In-containing balancer types disclosed the chromosomal location of a given w+ insert and produced balanced transgenic stocks. (The second chromosome balancer specified above is hereafter called CyO; and the third chromosome balancers are called TM3 and TM6.)
To induce HR, transgenic lines carrying the pW25/cry insert on the X or second chromosome were employed. Such transgenics were crossed to flies carrying FLP recombinase and I-SceI endonuclease (both under control of a heat shock protein promoter) on the second chromosome (using the transgenic types and the scheme reported in Rong and Golic 2000). {This strain is formally designated y w (v) P[ry+, 70FLP]4 P[v+, 70I-SceI]2B Sco/S 2 CyO.} Progeny were heat shocked for 3 days at 37° for 1 hr per day to induce FLP and I-SceI production. Among the resulting adults females were selected, because targeting-recombination frequencies are much higher in the germline of such Drosophila compared with males (Rong and Golic 2000). Females without CyO (therefore carrying FLP recombinase and I-SceI endonuclease) potentially carry gametes in which HR occurred between the pW25/cry insert and the cry locus. Certain such females, whose eyes were either mosaic for w+ expression or were completely w (due to the high rate of induced somatic excision and thus loss of the transgene), were selected for crossing to males carrying constitutively active FLP recombinase homozygous on the second chromosome (w1118 P[ry+, 70FLP]10). From among the progeny of this cross, flies with non-mosaic w+ eye color were selected, signifying HR involving the cry insert (the pertinent excised and then re-inserted DNA fragment lacks FRT sites and therefore cannot be excised by FLP). The chromosomal locations of such new insert sites were determined by crossing the w+ flies just indicated to y w CyO/Bl and y w TM3/H or w TM3/TM6. As above, the logic of balancer/dominant inheritance revealed which of these final stocks entailed re-insertion into chromosome 3, where the normal cry gene is located (Stanewsky et al. 1998).
Total replacement of cry+ in the four recombinant lines obtained (see results) was tested by PCR, using primers corresponding to intragenic cry sequences. One primer pair amplified part of the second cry exon: CRYf-2, 5′-TGGACTCGTTGCAGGACATC; CRYr-2, 5′-AGGAACATTTGGTAGGTCAGC. The second primer pair amplified a DNA fragment near the 3′ end of the third exon: CRYf-3, 5′-GTGTGCGGGGCGTTCAGATG; CRYr-3, 5′-GCCAGCGCAGACCGACCAAT. To gauge the integrity of these PCRs, control primers were also applied that corresponded to the third chromosomal Clock gene of (cf. Allada et al. 1998): CLKf, 5′-ATGATATTTTACTGCGTGAGGAT and CLKr, 3′-TCGCTGATGCCCATGTGAAAGT.
Additional genetic variants:
A deletion of cry+ and neighboring third chromosomal genes—Df(3R)Dl-Bx12 (Stanewsky et al. 1998)—was applied in certain tests by placing it in heterozygous conditions with a cry0 mutation. The “eye-blinding” norpAP24-null mutation (Pearn et al. 1996) was applied in singly mutant flies and by combining this X-chromosomal variant with three of the novel cry0 mutations. Flies carrying the latter along with the gl60j null allele, both of which are located within the right arm of the third chromosome, were generated by producing females homozygous for w and heterozygous in trans for the two mutations. These were mated to w gl60j males, and the progeny from 100 crosses were inspected for glass eyes and patches of red eye color; the latter marked the presence of w+ in the cry0 variants. These gl60j cry0 third chromosomes were extracted by crossing non-w, gl individuals to y w TM3/H, which led to the establishment of glass-eye CRY-less stocks.
To cope with genetic-background effects on rhythmic characters, material from the w1118 strain (Bloomington no. 6326, Hoskins et al. 2001), used as the source of control flies in several such phenotypic assessments, was introduced into cry0-containing flies. cry0 strains were crossed to w1118 followed by tracking w+ among the offspring; this outcrossing and re-extraction entailed six generations' worth of females heterozygous for cry0 and (w1118-derived) cry+. The resulting cry0 lines (called isoW) carry the w1118 mutation on the X chromosome, so their eye pigmentation is influenced only by mini-white+ presence (in this case the eye color is close to wild-type brick-red). Viability tests of the novel cry0 alleles were affected by performing the crosses specified in supplemental Table 1 at http://www.genetics.org/supplemental/.
Two extant transgenic strains were applied in neuro-anatomical tests of a cry0 mutation's (possible) effects on certain clock neurons in the brain and the neurites that extend from them. For this, a Pdf-gal4 driver (Park et al. 2000) was combined with a UAS-mCD8gfp drivee that encodes a membrane-bound derivative of green fluorescent protein (Lee and Luo 1999).
Genotyping elements of cry0's genetic background:
The rhythm-related timeless (tim) and jetlag (jet) genes exist in variant forms among certain strains of D. melanogaster (Koh et al. 2006; Peschel et al. 2006). Certain combinations of tim and jet variants can influence the flies' light responsive in chronobiological contexts (Peschel et al. 2006). Against this background, PCR was used to determine the tim alleles harbored by a cry0 stock used in phenotype analyses. Amplification of the requisite tim fragment—corresponding to the sites of alternative translation-initiating codons (Rosato et al. 1997; Tauber et al. 2007)—used these primers: 5′, 5′-AGATACCGCGCAAATGGCTAAGAAG and 3′, 5′-GGTGCGCAGTGTTTGGTCCTCATA. For amplification of a jet fragment, which includes the sites where mutations have been recognized at this locus (Koh et al. 2006; Peschel et al. 2006), the primers were: 5′, 5′-CACTGCTGGCCAACAACAAGAAAC and 3′, 5′-TGCACGCCATAGTCGGAGATAGC.
The H–B eyelet in D. melanogaster (see Introduction) expresses Rhodopsin genes called Rh5 and Rh6 (Malpel et al. 2002). In some strains of this species, Rh6 is a null variant: a 19-bp deletion that causes a stop codon to come in frame, within a portion of the coding sequence corresponding to the fifth transmembrane domain of the Rh6 polypeptide (Cook et al. 2003). Therefore, an Rh6 fragment, encompassing the region where this intragenic deletion lies, was amplified using these primers: 5′, 5′-CAAGGACTGGTGGAACAGGT; and 3′, 5′-GTTCATCTTCTTCGCCTGCT. It is notable that this Rh6-null mutation is harbored by certain w1118 stocks, which are used in many bio-genetic studies of D. melanogaster; this is also the case for a y cn bw sp stock from which DNA was taken to sequence the genome of this species (see the relevant BDGP subsite at http://flybase.bio.indiana.edu/). The expressible version of this Rhodopsin gene's sequence was initially reported by Huber et al. (1997).
The period gene has long been known to vary according to a Thr-Gly-repeat-encoding sequence in the middle of the gene. Such repeat-length variants are segregating in laboratory stocks (e.g., Yu et al. 1987) and in natural populations (e.g., Costa et al. 1992). Variation of the latter kind prompted tests of temperature compensation of the different per-isoallelic types (Sawyer et al. 1997). Because some of the phenotyping of currently applied strains involved varying the thermal condition, amplification of the relevant per fragment (taken from cry0-containing flies) was performed using these primers: 5′, 5′-GAGGGCAGCGGGGGCAGTG and 3′, 5′-GAGCAGGGATTCGGTCAGC.
The various amplicons produced as described in the foregoing passages were gel purified and directly sequenced.
Monitorings and analyses of whole-animal rhythmicities:
D. melanogaster were raised on a cornmeal/sugar/yeast/agar medium (supplemented with the mold inhibitor Tegosept) at 25° in 12:12 LD cycles and collected as 2–5-day-old adults. Locomotor activity of males was recorded using the Drosophila Activity Monitor system (DAM 5, Trikinetics, Waltham, MA). Flies had their locomotor movements recorded automatically for 5 days in LD (light intensity 800–1000 lux) and, in some tests, were switched to conditions of either LL or DD for at least 10 further days in a given run. In an LD-shift experiment, flies were entrained for 5 days in 12:12 LD, during the last one of which the onset of L was delayed by 8 hr, followed by 8 days of locomotor monitoring postshift (as in Helfrich-Förster et al. 2001). These data were plotted as actograms and average-activity histograms, as in Stanewsky et al. (1998). In another kind of re-entrainment experiment, flies were monitored for 5 days in the usual LD condition, then switched to a regime that entailed 9:9, 13:13, or 14:14 LD cycles (using equipment described in Mealey-Ferrara et al. 2003 re 12:12 to 13:13 shifts) for 7–8 days; at the end of the final (new, non-24-hr) LD cycle, free-running behavior was monitored for 4–6 days in DD. In certain photic conditions to which flies homozygous for cry0 were exposed, the ambient (constant) temperature was 18° or 29° instead of 25°.
Most of the locomotor analyses were performed using MATLAB-based software, which was developed for chronobiological purposes by Levine et al. (2002a,b). For flies that displayed two separate rhythmic components, by inspection of “actogram” plots of their locomotor cycles, autocorrelation analyses and MESA's failed to extract the “biperiodic” nature of these behaviors (see Figure 3 in results for an example). Therefore, each of the period values was determined by placing a ruler across the locomotor “onsets” that occurred on successive days. The slope defined by optimal placement of the straight-edge specified the free-running period. These semi-subjective “analog” determinations of periodicity were effected in an observer-blind manner, vis-à-vis the fly's genotype.
Figure 3.—
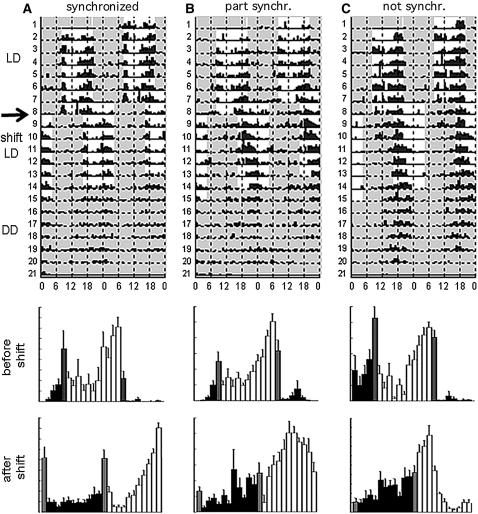
Locomotor behavior of norpAP24 cry0 flies after an abrupt LD shift. Top-row data: double-plotted actograms of representative flies behaving in 12-hr:12-hr LD cycles, then responding or not (as the case may be) to an 8-hr-delayed LD regime; the final portion of each behavioral record plots locomotor events occurring after the flies proceeded into DD (after the postshift LD regime). The arrow indicates when the LD-cycles were shifted. Plots at the bottom: “average activity” of flies before and after the photic shift, depicted in histograms that display the LD data only (white bars, locomotion in L; black, in D; gray, time bins during which the photic regime was changed from L to D or vice versa); a given bar shows the mean number of locomotor events (± SEM), per time bin per day for each of the three individuals. (A) A doubly mutant fly that adjusted its locomotion to behave in synchrony with the postshift LD cycle; note that there are clear behavioral “anticipations” of the L-to-D transitions (pre- and postshift), visible in the histograms. (B) A partially resynchronized double mutant whose daily peaks of locomotion got partly resynchronized (part synchr.), such that the phase of postshift locomotion was between the environmental phases of the old and the new LD regime 6 days after the shift. (C) A norpAP24 cry0 fly whose locomotor components did not get resynchronized (not synchr.), remaining in phase with original LD regime; note that the evening locomotor peak in new LD regime is similar to the preshift one (in the histograms), except that flies are active mostly in afternoon in the first LD regime, while this activity happened to occur in the “morning” in the new LD cycles.
Eclosion monitoring was performed as in Konopka et al. (1994), as modified slightly by Mealey-Ferrara et al. (2003). Key features of the procedures: The animals were raised in a 12:12 LD regime (throughout development); numbers of adult flies emerging per time unit were automatically counted, first for 1–2 days in 12:12 LD, then continuing in that condition or shifted to DD or to LL (light intensity at the level of the eclosion disc: 200 lux).
Luciferase reporting of clock-gene cycling:
The transgene called BG-luc, which encodes the N-terminal 2/3 of PER fused in frame to a firefly luciferase-encoding sequence (Stanewsky et al. 1997, 1998), was applied in combination with two of the newly created cry0 mutations. To obtain meiotic recombinants, cry0 flies were crossed to BG-luc ones, and the resulting trans-heterozygous females were mated to y w TM3/H males. Progeny from 40 crosses were visually inspected for eye color, whereby the w+ markers contained in both BG-luc and the cry0 allele combine their effects to produce a darker eye color than that exhibited by either single genotype. Recombinants recognized accordingly were crossed to y w TM3/H to establish balanced BG-luc cry0 stocks. These lines were verified by PCR for the presence of cry0 (primers: CRYf-2 and CRYr-2; CRYf-3 and CRYr-3, as above). Flies homozygous for both variants, along with BG-luc cry+ controls, were raised at 25° in 12:12 LD cycles and collected as 2–5-day-old adults. To assess LUC cycling in isolated tissue specimens, antennae or wings were pulled off adults (as in Plautz et al. 1997 and Levine et al. 2002b). Antennae and wings were chosen because BG-luc cycling was observed in these tissues homozygous for cryb (Levine et al. 2002b). Immediately postsurgery, specimens were placed in a luciferin-containing solution [insect tissue-culture medium (HyClone, Logan, UT) with 10% FBS (Invitrogen, Carlsbad, CA), 1% luciferin (Promega, Madison, WI), 0.5% insulin (Sigma, St. Louis, MO), and 1% antibiotic–antimycotic solution (GIBCO/Invitrogen)]. This material was distributed in 96-well plates, which were inserted into a Packard-Topcount Multiplate Scintillation counter (Perkin-Elmer, Waltham, MA). A given luminescent-monitoring run (cf. Krishnan et al. 2001; Levine et al. 2002b) proceeded for 5–7 days in 12:12 LD cycles (25°), over the course of which luminescence-based counts were accumulated every 30 min. The resulting data sets were analyzed as in Levine et al. (2002a,b).
Histology:
To visualize the axons projecting from “clock neurons” (known as LNv cells) (Hall 2003), brains from y w Pdf-gal4/UAS-mCD8gfp cry01 or 02, in parallel with cry+ controls, were dissected in PBS and immediately imaged on a Leica laser scanning confocal microscope TCS SP2 (Leica, Wetzlar, Germany).
RESULTS
Isolation of cry0 mutants:
To target the cryptochrome locus of D. melanogaster we choose an ends-out homologous recombination (HR) targeting strategy (Gong and Golic 2003). The construct for HR was designed such that the entire coding sequence of the cry+ allele would be replaced by mini-white+ (Figure 1). The pW25/cry vector contains two ∼3-kb fragments of third chromosomal DNA flanking the cry locus, along with w+ (between the segments of near-cry DNA) and FRT sequences (Figure 1). Transgenic lines were generated that contain this construct inserted at arbitrary genomic locations. To induce HR, whereby the cry-flanking/w+/cry-flanking DNA would be released from a “starting” transgene position and be able to replace the cry locus on chromosome 3, crosses were performed to elicit transgenically encoded FLP recombinase and I-SceI endonuclease activity in the female germline (see materials and methods). Approximately 700 food vials, each containing four of the relevant females and three males (carrying constitutively active FLP), were established; this led to the recovery of 55 potential HR lines. For these, chromosomal locations of the newly inserted w+ markers were determined, revealing that the eye-color marker had recombined into the third chromosome in four lines.
Focusing on the four candidates, PCRs were performed using primers derived from intra-cry locus sequences. Characterization of these amplicons revealed complete replacement of cry+ DNA in these four lines (Figure 1D). One line also contained another transposon-derived insert elsewhere on the third chromosome and therefore was not used further. The three variants for which cry+ was replaced by exogenous sequences were called cry–zero (cry0) mutants (cry01 through cry03). Several of the phenotypic tests of how these mutations influence rhythmic characters entailed usage of more than one cry0 strain (e.g., Tables 1 and 4). Drosophila homozygous for a cry0 mutation exhibited normal external morphology as well as robust male or female fertility. From viability-determining crosses the two kinds of tests showed emergence probabilities for cry0 flies to be normal (supplemental Table 1 at http://www.genetics.org/supplemental/).
TABLE 1.
Free-running locomotion, in DD or LL, at different temperatures
| Photic condition | 18°
|
25°
|
29°
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | N | N rhy (%) | Mean hr ± SEM | N | N rhy (%) | Mean hr ± SEM | N | N rhy (%) | Mean hr ± SEM | |
| DD | s-tim cry01 or 02 | 82 | 51 (62) | 23.7 ± 0.1 | 51 | 50 (98) | 23.7 ± 0.1 (82% of rhy) or 22.9 ± 0.1 (18% of rhy) | 55 | 52 (95) | 23.4 ± 0.1 |
| DD | s-tim cry+ | 16 | 15 (94) | 23.9 ± 0.1 | 14 | 14 (100) | 23.9 ± 0.0 | 29 | 29 (100) | 23.4 ± 0.1 |
| LL | s-tim cry01 or 02 | 89 | 55 (62) | 24.2 ± 0.1 | 74 | 70 (95) | 21.4 ± 0.0 and 25.4 ± 0.1 | 85 | 48 (57) | 20.1 ± 0.1 and 23.8 ± 0.2 |
| LL | ls-tim cry01 or 02 | ND | 67 | 57 (85) | 21.7 ± 0.0 and 25.4 ± 0.1 | ND | ||||
| LL | Df/cry01, 02, or 03 | ND | 63 | 58 (92) | 23.6 ± 0.1 (38% of rhy) 21.9 ± 0.2 and 25.0 ± 0.1 (62% of rhy) | ND | ||||
Flies were entrained under 12:12 hr light-dark cycling conditions for 5–6 days then released into constant conditions for 7 days of locomotor monitoring. s-tim and ls-tim, naturally occurring tim iso-alleles (Rosato et al. 1997; Peschel et al. 2006); Df, deletion of the cry locus and of neighboring third-chromosomal genes; N, numbers of tested flies or number rhythmic (rhy); Mean, average period determined by periodogram and verified by the straight-edge/slope procedure (see materials and methods); SEM, standard error of the mean; ND, not done (meaning that the LL monitorings of flies with genotypes in the bottom two rows were tested only at 25°). No differential effects on these characters were discernible with regard to cry0 derived from the separately numbered such strains (second column from the left).
TABLE 4.
Adult emergence of cry-mutant and cry+ flies in DD or LL
| DD
|
LL
|
|||||
|---|---|---|---|---|---|---|
| Genotype | N | Autocorr τ | MESA τ | N | Autocorr τ | MESA τ |
| cry02 | 3295 | 23.9 | 23.9 | 5121 | 23.9 | 23.5 |
| cryb | ND | ND | ND | 8050 | 24.6 | 24.8 |
| cry+ | 1488 | 23.0 | 23.6 | 6470 | AR | AR |
Eclosion profiles of cry0, cryb, and cry+ cultures were obtained from three independent monitorings, each of which encompassed two eclosion-monitor “discs” (cf. Konopka et al. 1994) for cry0 and cryb, and one for cry+ pupae. Data from all tests were pooled and analyzed together for a given genotype to estimate a combined period value (τ in hours), using autocorrelation (Autocorr) and MESA. AR, arrhythmic; N, total numbers of emergents; ND, not done.
Background genotypes of cry0 strains:
Several spontaneously occurring alleles of rhythm-related genes have been recognized in laboratory strains and in natural populations; these allelic types are not rarely encountered variants. They can influence chronobiological characters (Sawyer et al. 1997; Koh et al. 2006; Peschel et al. 2006), or one suspects that they would (cf. Malpel et al. 2002; Cook et al. 2003). Therefore, the relevant DNA sequences were characterized in our newly created cry0 lines. We focused on the four genes specified in materials and methods. To exclude possible interference of jetlag variants, we sequenced the pertinent part of the jet gene; none of the novel cry-null lines was found to harbor a “light-insensitive” jet mutation (viz. jetc or jetr, cf. Koh et al. 2006; Peschel et al. 2006). With regard to tim iso-alleles that produce either (relatively) short TIM protein or long-plus-short TIM in a given fly (Rosato et al. 1997; Tauber et al. 2007), we found that the Canton-S strain used in the current study carried ls-tim (encoding long and short-forms of TIM); and the w1118 strain harbors s-tim. But tim variation detected among the currently applied strains should not be problematic, because all of them are jet+ (Peschel et al. 2006). In this regard, cry0 combined with ls-tim did not promote behavior different from cry0 flies with the s-tim variant in LL (Table 1). Because expression of the Rhodopsin6 gene in the H–B eyelet is likely to influence circadian photosensitivity (see Introduction), we analyzed our stocks to ask whether any of them contain the Rh6-null mutation (cf. Cook et al. 2003). All the cry0 lines were found to be capable of encoding full-length Rh6. Given the temperature-dependent phenotype of cry0 flies (see Table 1), we sequenced the period gene with respect to a Thr-Gly (TG) repeat polymorphism. These numbers of TG pairs were found to be present in the cry0 strains: 17 TGs; the norpAP24 cry01-03 double mutants (whose X chromosome carries the per gene as well as the norpA one) and 20 TGs. Alleles of period that produce 17 or 20 TG pairs are among the commonly encountered types (Costa et al. 1992), bearing in mind the per17TG and per20TG types exhibit better temperature compensation of the clock's pace than do Drosophila containing other repeat-length PER protein types (Sawyer et al. 1997).
Locomotor rhythmicity in constant photic conditions affected by cry0:
Periodic locomotion of the new cry-mutant was robust in two free-running conditions: DD, in which nearly all wild-type Drosophila exhibit solid ∼24-hr periodicities (Hall 2003; Price 2005); and in LL, a condition that causes genetically normal flies to go arrhythmic (see Introduction). In DD, cry0 flies showed solidly rhythmic locomotion in almost all cases at 25° or 29°. Most period values were in the normal range, except that ∼20% of the cry0 individuals tested at 25° gave shorter than normal values (Table 1). When this mutant type was cooled to 18°, weak rhythmicity in DD was the norm, although the rhythmic individuals (60% of the total) gave normal period values (Table 1).
In LL, the great majority of cry0 individuals behaved rhythmically at 25°, a distinctly non-wild-type phenotype. This anomalous rhythmicity is similar to the behavior of the original cryb mutant in LL. However, that mutation, in some studies of it, led to simple outcomes of ∼24- or ∼25-hr free-running periods in LL (see Introduction). Here, most of the rhythmic individuals with cry-null genotype displayed two rhythmic components within a given behavioral record in LL (Yoshii et al. 2004). The average for the relatively short free-running periods (among these cry0 flies) was ∼21–22 hr; that of the longer was ∼25–26 hr (Table 1, example in Figure 2). A minority of the LL-rhythmic cry-null flies showed one periodic component per fly, ∼21–22 hr or ∼25–26 hr (legend to Table 1). Two split-rhythmic components had also been reported for cryb mutants in certain earlier studies involving locomotion in LL, but not in the majority of them (see discussion).
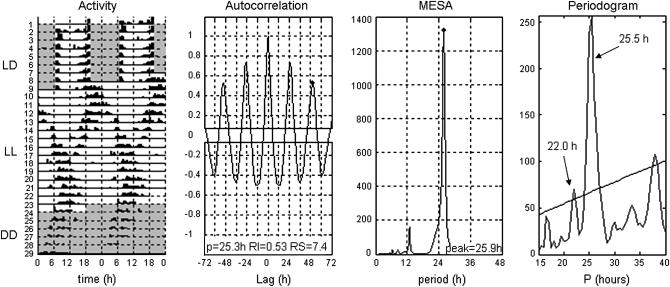
Figure 2.—
Locomotor behavior of cry0 in LD → LL → DD monitorings. The actogram (left) shows rhythmic behavior (Activity) in all three of the light regimes just indicated (respectively: LD cycles, LL, and DD). This cry0 fly displayed two rhythmic components during the LL component of the test, which disappeared after changing the conditions to DD. Autocorrelation, MESA, and Periodogram results were computed from the LL portion of the locomotor monitoring. The former two analyses revealed only one period >25 hr. (A given Lag in the correlogram is a value in hours that represents testing such numbers with respect to a “perfect” correlation between the behavioral data that were not lagged with respect to each other, which leads to a maximal peak height; in the MESA plot, the ordinate indicates spectral density, in arbitrary units, with regard to extraction of appreciably periodic components corresponding to hour values on the abscissa.) The Periodogram in the right-hand plot teased out the two free-running components (P on the abscissa = period) that are pointed to by arrows; each such hour value involved a significantly periodic component. [The Chi-square significance line crossing the periodogram, with respect to varying ordinate values in arbitrary units, corresponds to α = 0.05 (Sokolove and Bushell 1978).] Other cry0-derived actograms manifested two periodic components by inspection, but not all such behavioral records had the corresponding hour values extracted by Chi-square Periodogram analysis.
Results of testing flies heterozygous for a cry0 mutation and a deletion of the gene bear comment: Nearly all such Df/cry0 individuals (92%) behaved rhythmically in LL at 25°; ∼38% of rhythmic flies exhibited one periodic component (∼24 hr); whereas 62% gave two periodic components per individual (∼22 and ∼25 hr). Therefore, less splitting occurred when a cry-null mutation had its effects “uncovered” by the deletion, compared with the LL behavior of homozygotes; this could be explained in part by genetic-background differences. In this regard, the rhythmic characters displayed by Df/cry0 flies entailed much “noisier” periodic components compared with those influenced by homozygosity for cry0 mutations (Table 1). Therefore, specifying the precise proportions of Df/cry0's exhibiting a given single- vs. split-periodic attribute was problematical. (One reason for this could be result of the overall poor health of flies carrying the cry Df, as noted within supplemental Table 1 at http://www.genetics.org/supplemental/.)
We assessed effects of temperature changes on the bicomponent periodicity caused by a loss of cry function, because this gene has been previously connected with thermally varying situations in which two pacemaking functions operate within in a given animal (Rosato et al. 2001; Yoshii et al. 2004; Rieger et al. 2006). In LL at 29°, cry0 flies exhibited relatively large degrees of arrhythmicity (Table 1). When periodic components could be reliably analyzed, for ∼60% of the mutant types free-running in this heated condition, two periodicities were observed whose average values were ∼20 and ∼24 hr (Table 1). When LL behavior was monitored at 18°, again a higher percentage of arrhythmic behavior was observed (40%) compared with the 25° tests; and in this relatively cool condition, the rhythmic cry-null individuals yielded only one periodic component, ∼24 hr (Table 1).
cry0 flies display normal axonal projections from clock neurons that interconnect the brain hemispheres:
Splitting of free-running locomotor rhythmicity has been observed in other genetically defective circumstances. One such situation involves the effect of an eye-removing sine oculis (so) mutation, for which behavior in DD involved the frequent occurrence of two separate period values per mutant individual (Helfrich 1986). In a fair proportion of so brains, axons of certain “clock neurons” that project across the brain midline were found to be absent after an antibody against the Pdf gene product was applied (Helfrich-Förster and Homberg 1993). It was thus important to inspect the brain anatomy of cry0 adults. Furthermore, so-called “rhythm-functional” mutants can exhibit anomalies of clock-neuron axonal anatomy, as documented in Park et al. (2000). Examining whole-mounted brains homozygous for cry0, in a situation where homozygosity for cry0 was combined with a Pdf-gal4 transgene and UAS-mCD8gfp, disclosed no cry0-induced anatomical abnormalities (N = 40). The GFP signals projecting from LNv cells appeared in the typical wild-type pattern (cf. Hall 2003), which we also observed in control specimens (20 doubly transgenic brains carrying cry+).
norpA cry doubly null mutants exhibit severely defective resynchronization to altered LD cycles:
To create potentially circadian-blind Drosophila, we combined the norpAP24-null mutation with a cry0 one. The simultaneous effects of these variants were tested in two types of experiments. In one, we examined the ability of flies to resynchronize to new LD cycles after they were delayed by 8 hr. Singly mutant norpAP24 or cry0 individuals nicely re-entrained to the new light regime. But the great majority of double mutants failed to do so, in that their daily peaks of locomotion (typically occurring around lights-off and lights-on, respectively) continued to occur at the time of the photic transitions that were in operation before the 8-hr shift. In some cases the flies “partially entrained:” Six days after the photic shift, daily peaks of locomotion fell in between the phases expected for continuing entrainment to the old LD regime vs. re-entrainment to the new one (Table 2, Figure 3). It is likely that these doubly mutant individuals would have re-entrained if given enough time postshift; longer than normal times for such resynchronization of norpA cryb individuals were previously reported by Emery et al. (2000b) and Veleri et al. (2007).
TABLE 2.
Re-entrainment to delayed LD cycles
| Response categories %
|
||||
|---|---|---|---|---|
| Genotype | N | In synchrony with orig cycle | Resynchronized | Partially resynchronized |
| norpAP24 | 45 | 0 | 89 | 0 |
| cry01,02, or 03 | 21 | 0 | 95 | 0 |
| norpAP24cry01,02, or 03 | 115 | 37 | 15 | 43 |
Flies were entrained in 12:12 LD cycles then exposed to 8-hr delayed LD cycles. The ability of a fly to resynchronize to the new LD regime after 6 days was judged by inspecting that individual's actogram. Outcomes in the first data column here refer to flies whose locomotor components remained ostensibly in synchrony with the original (orig) LD regime (which could mean “no entrainment,” i.e., free-running behavior with ∼24-hr periodicity). The second data column documents flies whose activity was changed to conform with the postshift LD cycle, during at least 3 days after the 8-hr delay. Partially resynchronized: flies whose daily peaks of locomotion were judged to have conformed to neither the old nor the new LD regime, by 6 days after the shift. 11% of the norpAP24 flies and 5% of each of the norpAP24 cry0 and cry0 types became arrhythmic after phase shift (so the values in a given row do not sum to 100%). N, total number of flies rhythmic in initial LD. Doubly mutant individuals whose cry0 knock-out was derived from strain 01 exhibited the worst ability to resynchronize their behavior (postshift); but the cry02- or cry03-including flies of this type were only slightly better off.
In a second set of experiments, flies that had been put through 12:12-hr LD cycles were shifted to 9:9, 13:13, or 14:14 ones, to ask whether they would re-entrain to exhibit the appropriate 18, 26, or 28-hr periodicities. Examples from the 12:12 to14:14 test are in Figure 4. The singly mutant types routinely re-entrained to behave in synchrony with the novel non-24-hr environmental cycles (Table 3). norpAP24 cry0 flies, however, responded much less well: only 3–18% of the double mutants behaved in some synchrony with the new LD regime, depending on its duration. It is crucial to register that various proportions of the flies that shifted away from 12:12 cycles gave “ambiguous” results (see the right-hand portion of Table 3). Some individuals in this category yielded such sloppy behavioral records that no particular periodic component could be discerned. But several other of the ambiguously behaving norpAP24cry0 flies were solidly periodic and in no way had maintained close-to-24-hr cycle durations. Yet—as is detailed in the legend to Table 3–these double mutants manifested periods that differed from the new LD cycle durations by 1 to 4.5 hr (e.g., 43% of flies in the 12:12 to 9:9 experiment behaved with 22.5-hr periodicities in the 9:9 condition). Strikingly, ∼15% of norpAP2 cry0 flies in the 12:12 to 9:9 experiment yielded a roughly re-entrained periodic component together with ∼22–24-hr component (Table 3), within a given individual activity record of this type.
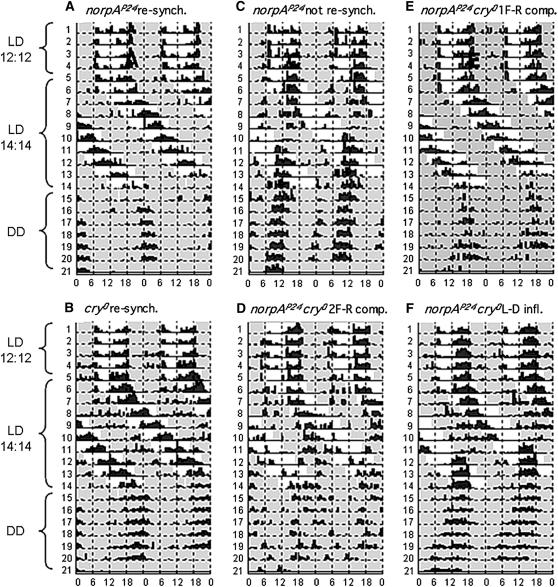
Figure 4.—
Locomotor behavior of singly-mutant cry0 and norpAP24 flies and of doubly mutant norpAP24 cry0 ones after a shift from 12:12 LD to a novel cycle duration. Each animal was released from the second L:D conditions to DD. (A and B) Singly mutant norpAP24 and cry0 individuals that resynchronized (resynchron.) to the new light regime, exhibiting ∼28-hr periods during 14:14 LD; signified by the upper-left to lower-right slant of the locomotor events during this photic condition (this being a typical long-period feature of an actogram component, vis-à-vis the two successive 12-hr days indicated on the abscissa). (C) A norpAP24cry0 fly that failed to resynchronize to 14:14 LD, maintaining free-running periodicity (∼24 hr) in both photic conditions. (D) A doubly mutant individual for which two rhythmic free-running (F-R) components (comp.) appeared after it was shifted to 14:14 LD; one component possessed a period <24 hr; the second component was ∼26 hr. (E) A double mutant that behaved with a single free-running component of ∼26 hr. (F) Example of a locomotor result influenced by the two mutations and (environmentally) by the “lights-on” transitions; this norpAP24cry0 individual displayed its highest activity during the light part of LD cycles but failed to re-entrain to 14:14 LD; a feature of the behavior plotted in this panel is “disconnected” locomotor during days 10–11 (arrow); for the remainder of the behavior occurring in 14:14 LD, this fly behaved with an ∼24-hr period.
TABLE 3.
Locomotor responses to shifts from 12:12 LD cycles to <24- or >24-hr cycles
| Behavioral categories after shift to 9:9 LD, mean period(s) ± SEM
|
|||||
|---|---|---|---|---|---|
| Genotype | N rhy/N tested (% rhy) | One periodic component: ∼24hr | One periodic component: ∼8 hr | Two periodic components | Ambiguous |
| norpAP24 | 39/44 (89) | NA | 18.1 ± 0.1 | NA | NA |
| cry01,02, or03 | 54/60 (90) | 23.5 ± 0.1 (11% of rhy) | 18.4 ± 0.2 (63% of rhy) | 18.0 ± 0.0 and 22.6 ± 0.3 (19% of rhy) | NA (7% of rhy) |
| norpAP24 cry01,02, or03 | 104/107 (97) | 23.6 ± 0.0 (69% of rhy) | 18.0 ± 0.9 (4% of rhy) | 19.8 ± 0.2 and 23.6 ± 0.1 (15% of rhy) | NA (12% of rhy) |
| Behavioral categories after shift to 13:13 LD, mean period(s) ± SEM
| |||||
|---|---|---|---|---|---|
| Genotype | N rhy/N tested (% rhy) | One periodic component: ∼24 hr | One periodic component: ∼26 hr | Two periodic components | Ambiguous |
| norpAP24 | 20/21 (95) | NA | 26.0 ± 0.0 (100% of rhy) | NA | NA |
| cry01or02 | 43/43 (100) | NA | 25.8 ± 0.0 (100% of rhy) | NA | NA |
| norpAP24 cry01or02 | 38/38 (100) | 24.2 ± 0.1 (42% of rhy) | 25.6 ± 0.1 (18% of rhy) | 22.4 ± 0.1 and 25.1 ± 0.2 (13% of rhy) | NA (26% of rhy) |
| Behavioral categories after shift to 14:14 LD, mean period(s) ± SEM
| ||||||
|---|---|---|---|---|---|---|
| Genotype | N rhy/N tested (% rhy) | One periodic component ∼24 hr | One periodic component ∼28 hr | Two periodic components | One periodic component with phase shift | Ambiguous |
| norpAP24 | 19/21 (91) | NA | 27.8 ± 0.1 (100% of rhy) | NA | NA | NA |
| cry01,02, or03 | 22/23 (96) | NA | 27.7 ± 0.1 (100% of rhy) | NA | NA | NA |
| norpAP24 cry01,02, or03 | 122/129 (95) | 24.2 ± 0.1 (20% of rhy) | 27.0 ± 0.0 (3% of rhy) | 23.5 ± 0.1 and 26.1 ± 0.1 (28% of rhy) | 25.7 ± 0.1 (13% of rhy) | NA (36% of rhy) |
Flies were exposed to 12:12 LD cycles for 5 days, then shifted to the novel LD cycles indicated for 7–8 days, followed by monitoring locomotion in DD (data from the latter condition not tabulated, but see Figure 4). Each postshift locomotor record was subjected to observer-blind analysis to evaluate its periodic component(s), if any, including whether or not the fly had resynchronized to the new cycle duration. Periods were determined by the straight-edge slope procedure noted in materials and methods.
Examples of behavioral responses in the 12:12 →14:14 test are in Figure 4. The “one periodic component with phase shift” outcome refers to the following: flies that displayed one periodic component, running with a certain period (always <28 hr) until the time when such individual flies “should” be active in the dark if their behavior continued with the same period; but starting at that point, an individual behaving in this category was not active during the entire 14 hr of DD; after lights-on, locomotion commenced again and continued to be periodic, although this new component was shifted relatively to the first such component (see Figure 4F).
Ambiguous, referring either to (i) locomotor patterns that were so messy (involving, for example, three putatively separate rhythmic components per actogram) that no proper categorization was possible; or (ii) to solid and simple periodic postshift outcomes that were judged not to be near enough to either 24 hr or to the novel LD cycle durations; specifically, in the 9:9 test, 3 of the 12 NA flies gave 22.5-hr periods; in 13:13, 7 of 10 NA's gave 25-hr periods; in 14:14 out of 44 NA's 1 fly gave a 25-hr period, 3 gave a 25.5-hr period, 19 gave a 26-hr period, and 12 gave a 26.5-hr period; NA, not applicable (meaning either that no behavioral outcomes fell into that category or that some did, but a particular period value could not be specified because of ambiguities among individuals); rhy, rhythmic; SEM, standard error of the mean.
Against a backdrop of the modest ability of gl60j cryb flies to synchronize their behavior to 26-hr LD cycles (Mealey-Ferrara et al. 2003), our tests of the newly created gl60j cry0 double mutant showed that it exhibited weak rhythmicity in general terms; several such individuals were arrhythmic altogether (data not shown). Among the numerical read-outs of these locomotor tests was an indication of poorly periodic behavior in 12:12 LD—signifying that these double mutants could not entrain to such “normal” cycles or that they were exhibiting sloppy free-run rhythmically in that condition. Thus it was not possible to assess whether re-entrainment to the newly imposed LD cycles was occurring in flies suffering the simultaneous effects of a cry-null and a glass mutation.
cry mutants display periodic eclosion in all photic conditions, including LL:
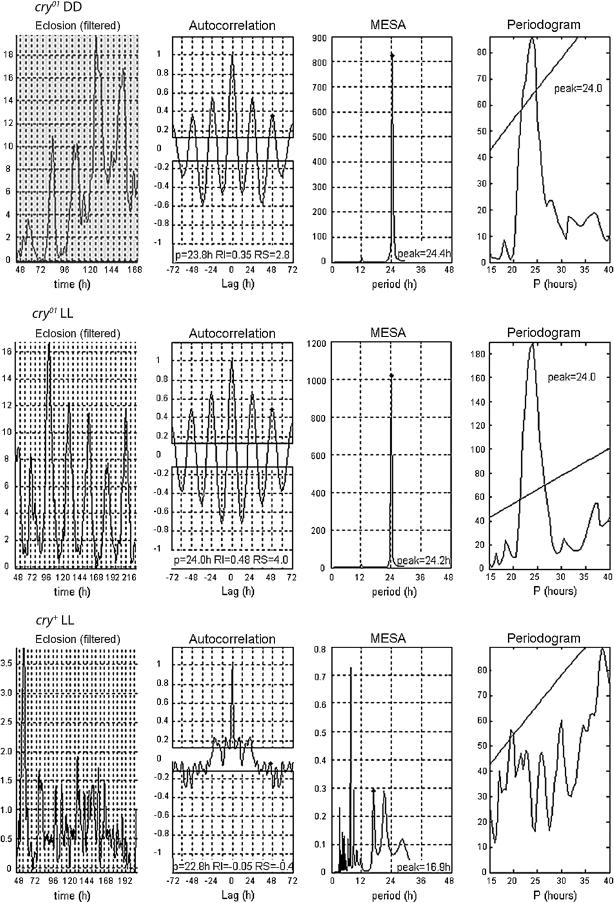
Assays of eclosion rhythmicity affected by the novel cry mutations (Figure 5) showed that, after cultures were entrained by LD cycles, cry01 and cry02 adults emerged rhythmically in DD with periodicities similar to control values (Table 4). In LL, which causes eclosion of genetically normal Drosophila to be arrhythmic (Chandrashekaran and Loher 1969; Winfree 1974; Sheeba et al. 1999), we encountered a novel chrono-genetic phenomenon: cry0 cultures eclosed rhythmically; these LL period values were similar to those derived from cry0 or cry+ animals emerging in DD (Figure 5, Table 4). Because no rhythm mutants have been studied for eclosion occurring in LL, we tested cryb in that condition. This mutant type also exhibited anomalously periodic adult-emergence in LL (Table 4).
Figure 5.—
Free-running periodic emergence of cry0 flies into adulthood. Late-developing cry0 cultures displayed periodic adult emergence in both DD and LL. The left-hand plots show adult-emergence profiles after application of a bandpass filter, which removed periodic components <4 and >40 hr (cf. Levine et al. 2002a). The other plots show analytical outcomes, the conventions for which (e.g., ordinate labels) are as in Figure 2. Wild-type cry+ cultures, which routinely eclose periodically in DD (e.g., Table 4), gave an eclosion profile in LL for which daily peaks damped out after ∼48 hr of being shifted to this condition.
A previous test of cryb eclosion in DD showed such cultures to emerge rhythmically in that condition (Mealey-Ferrara et al. 2003), whereas this mutant was reported to eclose arrhythmically in LD (Myers et al. 2003). In the current study, monitoring cry0 eclosion in the latter condition led to vigorously periodic adult emergence, although the average peak time (per LD day) was slightly before dawn, as opposed to slightly after that time for cry+ control cultures or cryb mutant ones (supplemental Figure 1 at http://www.genetics.org/supplemental/).
cry0's effect on luciferase reporting of clock-gene cycling in peripheral tissues:
Krishnan et al. (2001) and Levine et al. (2002b) previously reported the effects of the cryb mutation on period and timeless gene expression within isolated peripheral Drosophila tissues. That seminal mutation significantly decreased proportions of rhythmic specimens in LD and DD (compared with the usual values given by various tissue types), with these exceptions: Antennal rhythmicity was only partially degraded, and wing rhythmicity was barely affected by cryb (Levine et al. 2002b). To ask whether elimination of CRY would lead to more severe arrhythmia in isolated tissues, we monitored per-luc luminescence fluctuations in wings and antennae under LD conditions. Luciferase-reported cycling was reduced by the effects of cry0 on both appendage types, but an appreciable proportion of specimens displayed rhythmicity: approximately two-thirds of the wings and approximately one-third of the antennae were rhythmic (supplemental Table 2 at http://www.genetics.org/supplemental/).
DISCUSSION
The aim of this work was to generate a true cryptochrome-null mutation in Drosophila and assess the spectrum of rhythm-related abnormalities that could be caused by an absence of CRY protein. These cry0 mutants were generated by an HR tactic that removed the entire CRY-encoding region of chromosome 3; this resulted in a handful of knock-out lines, all of them in-principle identical. Three of these strains and their derivatives were studied in detail.
Two-component free-running rhythms induced by cry mutations:
One striking phenotype of the original cry mutant is behavioral rhythmicity in LL (e.g., Emery et al. 2000a), a condition that causes wild-type flies to exhibit aperiodic locomotion (Konopka et al. 1989). When cry0 flies were exposed to this photic condition (LL), they also displayed rhythmic behavior, albeit with properties different from those usually described for cryb (the initially isolated mutant). Nearly all cry0 individuals displayed two free-running periodic components in LL. A similar phenotype was described for cryb by Yoshii et al. (2004) and by Rieger et al. (2006), with the following provisos: When the photic conditions entailed very dim LL (<1 lux in Yoshii et al. 2004), a single free-running locomotor component was observed, whereas two such components were displayed by this mutant as the intensity of LL was raised (>10 lux). Such cryb-induced “rhythm dissociation” was not observed by other investigators (Emery et al. 2000a; Helfrich-Förster et al. 2001; Mealey-Ferrara et al. 2003), even though they applied high light intensities for which behavioral “splitting” was observed by, for example, Yoshii et al. (2004). A mnemonic device for the ensemble of these results is that cryb flies exhibit splitting when LL is in the range of ∼10–400 lux, whereas cry0 ones give this response even at a higher light intensity.
What causes a CRY-less fly, and a CRY-depleted one in certain circumstances, to exhibit bicomponent periodicities? Such splitting could have to do with separate free running-periods by which the clockworks might be operating in different brain cells, or even arise from two rhythm components being present in one such cell, as occurs in unicellular Gonyaulax (Roenneberg and Morse 1993). A related possibility is that the locomotor splittings in question are not per se the result of removing or depleting CRY; instead, these complex rhythmic components could be part of Drosophila's “basic” chronobiological capacity (cf. Helfrich-Förster 2001) and are uncovered by the effects of cry mutations. In other words, relatively fast- and slow-running clocks would (also) be harbored within wild-type brains, but these different paces are ordinarily “processed” to result in overall ∼24-hr periodicities. One speculative function for the naturally occurring two-pacemaker situation is that the <24-hr and >24-hr components are involved in temperature compensation: Relatively cool or warm thermal conditions would have the opposite effect on each component (speeding up one while slowing down the other), resulting in a final read-out near 24 hr. Our temperature-varied experiments, however, do not support this notion, at least not in the case of 29° LL locomotor monitorings, for which both free-running components manifested the apparent effects of fast-running circadian pacemakers.
The abovementioned CNS defect induced by sine oculis (so) mutations, which can cause individual Drosophila to display more than one circadian component in DD, leads to the idea that an inter-pacemaker communication defect is among cry0's rhythm-related defects. That is, the insensitivity of deep-brain photoreceptors (cf. Emery et al. 2000b) to LL in a cry mutant could be connected with a failure of these CRY-less neurons to mediate synchrony between bilaterally symmetrical pacemaker cells, by analogy to the correlation between so-induced behavioral splitting (Helfrich 1986) and the frequent absence of an inter-pacemaker anatomical connection in this mutant type (Helfrich-Förster and Homberg 1993). Wild-type flies in DD could not, of course, enjoy any photically controlled communication between such neurons in each of the two brain hemispheres. But when such cry+ individuals get exposed to LD cycles (or at least LD transitions) prior to proceeding into DD, operations of these circadian neurons may gear-up (thanks to CRY-mediated photoreception within the brain), to sustain intra-brain synchrony-related functions. Squarely in this regard, some genetically normal Drosophila exhibit the behavioral effects of (apparent) internal desynchronization after lengthy sessions in DD, similar to so-induced splitting in that condition or cry-induced splitting in LL (Helfrich-Förster 2000).
Entrainment to LD cycles and resynchronization to shifted photic conditions:
The cryb mutation causes rhythm-related entrainment problems, especially in combination with a given visual-system mutation: no-receptor-potential-A or glass (Stanewsky et al. 1998; Helfrich-Förster et al. 2001). When a probable norpA-null mutation (allele P41) was added to a cryb-homozygous genetic background, certain proportions of the doubly mutant flies were capable of re-entraining their locomotor cycles. For example, norpAP41 cryb individuals were asked to resynchronize from LD cycles (L = 650 lux white light) to 4-hr-shifted ones (L = 16 lux blue); 27% of the flies readjusted their locomotor cycles accordingly (Stanewsky et al. 1998). Our findings, involving a confirmed norpA-null allele (P24) combined with cry0, revealed that only 15% of the double mutants re-entrained fully to an 8-hr LD shift (both L's = 850 lux white); this lower proportion (compared with 27% in Stanewsky et al. 1998) may have occurred because of the more abrupt LD shift employed in the current experiments. The same kind of test was performed by Helfrich-Förster et al. (2001) on glass-null cryb double mutants, 0% of which individuals resynchronized their behavior after 8-hr alterations of LD cycles. The gl-with-cry case will resurface shortly below.
Another prior related study asked norpAP41 cryb double mutants to adjust their locomotion away from being synchronized with 12:12 LD (L = 850 lux white), such that they would operate on the same time scale of 13:13 cycles. Approximately 40% of the individuals ended up entraining to the latter cycle, shifting from displays of ∼24-hr periods to ∼26-hr ones (Mealey-Ferrara et al. 2003). Less impressively, at most 18% of norpAP24cry0 flies exhibited resynchronization to the second kind of cycle in our corresponding experiment, but <5% did so in the 12:12 → 9:9 or the 12:12 → 14:14 tests (Table 3). This means that this double mutant type readjusted its behavior most readily when such correction involved only a 2-hr stretch (12:12 → 13:13). Combining a glass mutation with the latter kind of cry was once said to create the most severe circadian-blinding genotype, soley on the basis of experiments involving abrupt 8-hr shifts of LD cycles (Helfrich-Förster et al. 2001). However, in conditions comparable to our current 12:12 → novel-cycle tests (whereby L within a given LD period was 850 lux) ∼55% of gl60j cryb individuals resynchronized to the new environmental cycle (Mealey-Ferrara et al. 2003).
Overall, then, mutant Drosophila in which no light-induced responsiveness of external photoreceptors is possible and who are (at last) thoroughly devoid of CRY protein exhibit among the poorest instances of entrainment to photic cycles and resynchronization to adjusted LD cycles. However, behavioral readjustments do occur for varying proportions of the double mutants (as summarized in this subsection). Nonetheless, the simultaneously norpA- and cry-null genotype has more severe consequences than does a norpA-mutated, cry-missense combination.
Temperature responsiveness of the novel cry-null mutant:
Although cry0 flies displayed normal behavior in DD at 25°, their rhythmicity was affected when locomotor cycles were running at 18° or 29° (Table 1, which documents reduced percentages of rhythmic individuals in relatively cool or warm conditions). A similar effect was observed in LL, again at temperatures lower or higher than the apparent optimal one (25°). These data suggest further that, in addition to its role in photic entrainment, CRY is also involved in central pacemaking as such (cf. Ivanchenko et al. 2001; Krishnan et al. 2001; Collins et al. 2006; Stoleru et al. 2007). In other words, cry+ seems to be important for the most favorably temperature-compensated behavior, although not in this case speaking to the maintenance of ∼24-hr periodicities in different thermal conditions (Sawyer et al. 1997); instead, for basic rhythm maintenance when the temperature strays rather far from 25°.
Synchronization of Drosophila late in development for periodic eclosion:
It is necessary to synchronize fruit-fly cultures with environmental inputs if pharate adults are to emerge rhythmically, because this circadian periodic phenotype is a population phenomenon. A corollary, in a way, is that eclosion occurs arrhythmically if pupae are exposed to LL (Chandrashekaran and Loher 1969). And because that condition militates against CRY accumulation (Emery et al. 1998; Lin et al. 2001), one would imagine that cry-depleting mutations should induce aperiodic eclosion, if this protein is essential for its photic entrainment. Fulfillment of this tacit prediction was reported by Myers et al. (2003) for cryb eclosion, which was said to be arrhythmic even in LD. In contrast, Mealey-Ferrara et al. (2003) showed that that mutation allows for LD entrainment of cultures such that they exhibited solidly periodic eclosion in (subsequent) DD. Another matter addressed by the study just cited: the fact that norpA+ function contributes to synchronization of clock-protein cycling in developing Drosophila; this phenomenon operates in larval brain neurons (Kaneko et al. 2000). But the simultaneous presence of a norpA mutation and cryb left developing animals synchronizable for periodic eclosion if they were exposed to LD after the larval stage before proceeding into DD (Mealey-Ferrara et al. (2003). The same photic regime was applied in elements of the current eclosion tests. Their results imply that what is necessary, or at least sufficient, for LD synchronization of pupae entails a mysterious photoreceptive process that is both NORPA- and CRY-independent (see Conclusions).
Elements of the current findings shed further light on CRY's contribution to the control of periodic eclosion, because adult emergence of cry0 flies was found to be clearly rhythmic in LD (supplemental Figure 1 at http://www.genetics.org/supplemental/). This could imply that CRY has no influence of synchronization of late-developing Drosophila. But that supposition is belied by a newly uncovered effect of cry mutations—which is that depletion or elimination of the encoded protein renders the animal insensitive to the arrhythmia-inducing effects of LL (Figure 5).
Conclusions:
Previous studies of cryptochrome mutants revealed a role for the light-absorbing substance it encodes in photic-input and peripheral clock mechanisms but left many questions unanswered. To address these issues, which mostly involve ambiguous effects of the cryb mutation, we generated the first true cry-null flies and subjected them to the requisite array of chronobiological tests. Along the way, we accounted for possibly confounding genetic-background effects and neuro-developmental defects (none and none). We found that cry0 flies displayed more severely abnormal light-response phenotypes than do cryb ones, bolstering the frequently discussed supposition that residual CRY function lurks within chronobiologically meaningful tissues of cryb animals.
Our analysis also lends credence to the existence of CRY-independent pathways that are responsible for the residual photoreceptive capacities previously observed in norpAP41 cryb flies (or gl60j cryb ones) and the maintenance of peripheral-clock functioning in certain appendage types set up to express the effects of cryb. The “extra” pathway in question, the one functioning somewhere within the intact animal, may be headed-up by photoreceptive CNS neurons (N's) located in the dorsal (D) brain (see Helfrich-Förster et al. 2001 and Veleri et al. 2003 for the relevant results and discussions). A seemingly remarkable implication of this presumption is that the dorsal neurons in question (called neuronal cluster DN1) would possess photoreceptive capacities that are dependent neither on CRY (this report), nor on any known form of Drosophila rhodopsin. This notion should be considered in context of the conventional wisdom about the latter such substances, whereby the norpA gene product would transduce the results of all rhodopsin-mediated light absorption (Pak and Leung 2003). However, recall our mentioning the discussion of rhodopsin-containing H–B eyelet cells functioning independently of norpA function insofar as circadian photoreception is concerned; although this supposition was based mainly on the fact that a norpA cry double mutant, for which neither mutation was known to be a null, can “still entrain” (Veleri et al. 2007; cf. Stanewsky et al. 1998). Nevertheless, and thus assuming that circadian photoreception in Drosophila can occur in a manner that is both NORPA- and CRY-independent (this report), what if the abovementioned DN1 cells indeed are CNS photoreceptors? Their position is not so deep into the brain, with reference to the CRY-containing lateral neurons (Emery et al. 2000b). The dorsally located neurons could possess an opsin that does not need the norpA-encoded enzyme for the photransduction process in question (Chang and Reddy 2000).
As a final point, we have obtained novel results suggesting that CRY plays a role in the maintenance of central clock function at temperature extremes, and that hypomorphic or null alleles of cry permit synchronized periodic eclosion of flies even in LL. The ensemble of these experiments advance our understanding of roles played by cryptochrome on behalf of circadian inputs and of pacemaker mechanisms, as well as establishing an important new genetic variant for future research.
Acknowledgments
We thank Yikang Rong and Kent Golic for providing strains that mediate homologous recombination of transgenes and the Bloomington Indiana Stock Center for supplying some of the basic stocks (genetically normal and mutant strains). We appreciate the assistance with Matlab applications furnished by Pablo Funes and the confocal help provided by Edward Dougherty. We are grateful for comments on the manuscript from Dennis C. Chang. This work was supported by grants from the National Institutes of Health (NS-44232 and GM-66778).
References
- Allada, R., N. E. White, W. W. So, J. C. Hall and M. Rosbash, 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93: 791–804. [DOI] [PubMed] [Google Scholar]
- Bi, X., and Y. S. Rong, 2003. Genome manipulation by homologous recombination in Drosophila. Brief Funct. Genomic Proteomic 2: 142–146. [DOI] [PubMed] [Google Scholar]
- Busza, A., M. Emery-Le, M. Rosbash and P. Emery, 2004. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 304: 1503–1506. [DOI] [PubMed] [Google Scholar]
- Chandrashekaran, M. K., and W. Loher, 1969. The effect of light intensity on the circadian rhythm of eclosion in Drosophila pseudoobscura. Z. Vergl. Physiol. 62: 337–347. [Google Scholar]
- Chang, H. Y., and D. F. Reddy, 2000. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science 290: 1978–1980. [DOI] [PubMed] [Google Scholar]
- Collins, B., E. O. Mazzoni, R. Stanewsky and J. Blau, 2006. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr. Biol. 16: 441–449. [DOI] [PubMed] [Google Scholar]
- Cook, T., F. Pichaud, R. Sonneville, D. Papatsenko and C. Desplan, 2003. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Devel. Cell 4: 853–864. [DOI] [PubMed] [Google Scholar]
- Costa, R., A. A. Peixoto, G. Barbujani and C. P. Kyriacou, 1992. A latitudinal cline in a Drosophila clock gene. Proc. R. Soc. Lond. Ser. B 250: 43–49. [DOI] [PubMed] [Google Scholar]
- Dissel, S., V. Codd, R. Fedic, K. J. Garner, R. Costa et al., 2004. A constitutively active cryptochrome in Drosophila melanogaster. Nat. Neurosci. 7: 834–840. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. C., J. J. Loros and P. P. Decoursey, 2004. Chronobiology: Biological Timekeeping. Sinauer Associates, Sunderland, MA.
- Emery, P., W. V. So, M. Kaneko, J. C. Hall and M. Rosbash, 1998. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95: 669–679. [DOI] [PubMed] [Google Scholar]
- Emery, P., R. Stanewsky, J. C. Hall and M. Rosbash, 2000. a Drosophila cryptochrome - a unique circadian-rhythm photoreceptor. Nature 404: 456–457. [DOI] [PubMed] [Google Scholar]
- Emery, P., R. Stanewsky, C. Helfrich-Förster, M. Emery-Le, J. C. Hall et al., 2000. b Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26: 493–504. [DOI] [PubMed] [Google Scholar]
- Frank, K. D., and W. F. Zimmerman, 1969. Action spectra for phase shifts of a circadian rhythm in Drosophila. Science 163: 688–689. [DOI] [PubMed] [Google Scholar]
- Gong, W. J., and K. G. Golic, 2003. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA 100: 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. C., 2003. Genetics and molecular biology of rhythms in Drosophila and other insects. Adv. Genet. 48: 1–280. [DOI] [PubMed] [Google Scholar]
- Helfrich, C., 1986. Role of the optic lobes in the regulation of the locomotor activity rhythms in Drosophila melanogaster: behavioral analysis of neural mutants. J. Neurogenet. 3: 321–343. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 2000. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster–sex-specific differences suggest a different quality of activity. J. Biol. Rhythms 15: 135–154. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., 2001. The activity rhythm of Drosophila melanogaster is controlled by a dual oscillator system. J. Insect Physiol. 47: 877–887. [Google Scholar]
- Helfrich-Förster, C., and U. Homberg, 1993. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J. Comp. Neurol. 337: 177–190. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster, C., C. Winter, A. Hofbauer, J. C. Hall and R. Stanewsky, 2001. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30: 249–261. [DOI] [PubMed] [Google Scholar]
- Hofbauer, A., and E. Buchner, 1989. Does Drosophila have seven eyes? Naturwissenschaften 76: 335–336. [Google Scholar]
- Hoskins, R. A., A. C. Phan, M. Naeemuddin, A. F. Mapa, D. A. Ruddy et al., 2001. Single nucleotide polymorphism markers for genetic mapping in Drosophila melanogaster. Genome Res. 11: 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, A., S. Schulz, J. Bentrop, C. Groell, U. Wolfrum et al., 1997. Molecular cloning of Drosophila Rh6 rhodopsin: the visual pigment of a subset of R8 photoreceptor cells. FEBS Lett. 406: 6–10. [DOI] [PubMed] [Google Scholar]
- Ivanchenko, M, R. Stanewsky and J. M. Giebultowicz, 2001. Circadian photoreception in Drosophila: functions of cryptochrome in peripheral and central clocks. J. Biol. Rhythms 16: 205–215. [DOI] [PubMed] [Google Scholar]
- Kaneko, M., M. J. Hamblen and J. C. Hall, 2000. Involvement of the period gene in developmental time-memory: effect of the perShort mutation on phase shifts induced by light pulses delivered to Drosophila larvae. J. Biol. Rhythms 15: 13–30. [DOI] [PubMed] [Google Scholar]
- Klarsfeld, A., S. Malpel, C. Michard-Vanhee, M. Picot, E. Chelot et al., 2004. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J. Neurosci. 24: 1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm, E., and H. Ninnemann, 1976. Detailed action spectrum for the delay shift in pupae emergence of Drosophila pseudoobscura. Photochem. Photobiol. 24: 369–371. [Google Scholar]
- Koh, K., X. Zheng and A. Sehgal, 2006. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 312: 1809–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, R. J., C. Pittendrigh and D. Orr, 1989. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J. Neurogenet. 6: 1–10. [DOI] [PubMed] [Google Scholar]
- Konopka, R. J., M. J. Hamblen-Coyle, C. F. Jamison and J. C. Hall, 1994. An ultrashort clock mutation at the period locus of Drosophila melanogaster that reveals some new features of the fly's circadian system. J. Biol. Rhythms 9: 189–216. [DOI] [PubMed] [Google Scholar]
- Krishnan, B., S. E. Dryer and P. E. Hardin, 1999. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400: 375–378. [DOI] [PubMed] [Google Scholar]
- Krishnan, B., J. D. Levine, K. S. Lynch, H. B. Dowse, P. Funes et al., 2001. A novel role for cryptochrome in a Drosophila circadian oscillator. Nature 411: 313–317. [DOI] [PubMed] [Google Scholar]
- Lee, T., and L. Luo, 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461. [DOI] [PubMed] [Google Scholar]
- Levine, J. D., P. Funes, H. B. Dowse and J. C. Hall, 2002. a Signal analysis of behavioral and molecular cycles. BMC Neurosci. 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, J. D., P. Funes, H. B. Dowse and J. C. Hall, 2002. b Advanced analysis of a cryptochrome mutation's effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F.-J., W. Song, E. Meyer-Bernstein, N. Naidoo and A. Sehgal, 2001. Photic signaling by cryptochrome in the Drosophila circadian system. Molec. Cell. Biol. 21: 7287–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpel, S., A. Klarsfeld and F. Rouyer, 2002. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development 129: 1443–1453. [DOI] [PubMed] [Google Scholar]
- Mealey-Ferrara, M. L., A. G. Montalvo and J. C. Hall, 2003. Effects of combining a cryptochrome mutation with other visual-system variants on entrainment of locomotor and adult-emergence rhythms in Drosophila. J. Neurogenet. 17: 171–221. [PubMed] [Google Scholar]
- Myers, E. M., J. Yu and A. Sehgal, 2003. Circadian control of eclosion: interaction between a central and peripheral clock in Drosophila melanogaster. Curr. Biol. 13: 526–533. [DOI] [PubMed] [Google Scholar]
- Ohata, K., H. Nishiyama and Y. Tsukahara, 1998. Action spectrum of the circadian clock photoreceptor in Drosophila melanogaster, pp. 167–170, in Biological Clocks: Mechanisms and Applications, edited by Y. Touitou. Elsevier Science B. V., Amsterdam.
- Pak, W. L., and H. T. Leung, 2003. Genetic approaches to visual transduction in Drosophila melanogaster. Recept. Channels 9: 149–167. [PubMed] [Google Scholar]
- Park, J. H., C. Helfrich-Förster, G. Lee, L. Liu, M. Rosbash et al., 2000. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc. Natl. Acad. Sci. USA 97: 3608–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S., and J. K. Lim, 1995. A microinjection technique for ethanol-treated eggs and a mating scheme for detection of germline transformants. Drosoph. Inf. Serv. 76: 197–199. [Google Scholar]
- Pearn, M. T., L. L. Randall, R. D. Shortridge, M. G. Burg and W. L. Pak, 1996. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J. Biol. Chem. 271: 4937–4945. [DOI] [PubMed] [Google Scholar]
- Peschel, N., S. Veleri and R. Stanewsky, 2006. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila's circadian clock. Proc. Natl. Acad. Sci. USA 103: 17313–17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz, J. D., M. Kaneko, J. C. Hall and S. A. Kay, 1997. Independent photoreceptive circadian clocks throughout Drosophila. Science 278: 1632–1635. [DOI] [PubMed] [Google Scholar]
- Price, J. L., 2005. Genetic screens for clock mutants in Drosophila. Meth. Enzymol. 393: 35–60. [DOI] [PubMed] [Google Scholar]
- Rieger, D., O. T. Shafer, K. Tomioka and C. Helfrich-Forster, 2006. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J. Neurosci. 26: 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz et al., 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg, T., and D. Morse, 1993. Two circadian oscillators in one cell. Nature 362: 362–364. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2000. Gene targeting by homologous recombination in Drosophila. Science 288: 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rosato, E., A. Trevisan, F. Sandrelli, M. Zordan, C. P. Kyriacou et al., 1997. Conceptual translation of timeless reveals alternative initiating methionines in Drosophila. Nucleic Acids Res. 25: 455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato, E., V. Codd, G. Mazzotta, A. Piccin, M. Zordan et al., 2001. Light-dependent interactions between Drosophila CRY and the clock protein PER mediated by the carboxy terminus of CRY. Curr. Biol. 11: 909–917. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Sancar, A., 2000. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Ann. Rev. Biochem. 69: 31–67. [DOI] [PubMed] [Google Scholar]
- Sawyer, L. A., M. J. Hennessey, A. A. Peixoto, E. Rosato, H. Parkinson et al., 1997. Natural variation in Drosophila clock gene and temperature compensation. Science 278: 2117–2120. [DOI] [PubMed] [Google Scholar]
- Sheeba, V., V. K. Sharma, M. K. Chandrashekaran and A. Joshi, 1999. Persistence of eclosion rhythm in Drosophila melanogaster after 600 generations in an aperiodic environment. Naturwissenschaften 86: 448–449. [DOI] [PubMed] [Google Scholar]
- Sokolove, P. G., and W. N. Bushell, 1978. The chi square periodogram: its utility for analysis of circadian rhythms. J. Theoret. Biol. 72: 131–160. [DOI] [PubMed] [Google Scholar]
- Stanewsky, R., C. F. Jamison, J. D. Plautz, S. A. Kay and J. C. Hall, 1997. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 16: 5006–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky, R., M. Kaneko, P. Emery, B. Beretta, K. Wager-Smith et al., 1998. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95: 681–692. [DOI] [PubMed] [Google Scholar]
- Stoleru, D., P. Nawathean, Mde. L. Fernandez, J. S. Menet, M. F. Ceriani et al., 2007. The Drosophila circadian network is a seasonal timer. Cell 129: 207–219. [DOI] [PubMed] [Google Scholar]
- Tauber, E., M. Zordan, F. Sandrelli, M. Pergoraro, N. Osterwalder et al., 2007. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316: 1895–1898. [DOI] [PubMed] [Google Scholar]
- Veleri, S., C. Brandes, C. Helfrich-Förster, J. C. Hall and R. Stanewsky, 2003. A self-sustaining, light-entrainable circadian oscillator in the brain of Drosophila. Curr. Biol. 13: 1758–1767. [DOI] [PubMed] [Google Scholar]
- Veleri, S., D. Rieger, C. Helfrich-Förster and R. Stanewsky, 2007. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates PER and TIM expression in Drosophila clock neurons. J. Biol. Rhythms 22: 29–42. [DOI] [PubMed] [Google Scholar]
- Venken, K. J. T., and H. J. Bellen, 2005. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat. Rev. Genet. 6: 167–178 (erratum: Nat. Rev. Genet. 6: 340). [DOI] [PubMed] [Google Scholar]
- Winfree, A. T., 1974. Suppressing Drosophila's circadian rhythm with dim light. Science 183: 970–972. [DOI] [PubMed] [Google Scholar]
- Yoshii, T, Y. Funada, T. Ibuki-Ishibashi, A. Matsumoto, T. Tanimura et al., 2004. Drosophila cryb mutation reveals two circadian clocks that drive locomotor rhythm and have different responsiveness to light. J. Insect Physiol. 50: 478–488. [DOI] [PubMed] [Google Scholar]
- Yu, Q., H. V. Colot, C. P. Kyriacou, J. C. Hall and M. Rosbash, 1987. Behaviour modification by in vitro mutagenesis of a variable region within the period gene of Drosophila. Nature 326: 765–769. [DOI] [PubMed] [Google Scholar]
- Zimmerman, W. F, and T. H. Goldsmith, 1971. Photosensitivity of the circadian rhythm and of visual receptors in carotenoid-depleted Drosophila. Science 171: 1167–1169. [DOI] [PubMed] [Google Scholar]