Abstract
The Pmr1 Golgi Ca2+/Mn2+ ATPase negatively regulates target of rapamycin complex (TORC1) signaling, the rapamycin-sensitive TOR complex in Saccharomyces cerevisiae. Since pmr1 causes resistance to rapamycin and tor1 causes hypersensitivity, we looked for genetic interactions of pmr1 with tor1. Deletion of TOR1 restored two wild-type phenotypes. Loss of TOR1 restored the ability of the pmr1 strain to grow on media containing 2 mm MnCl2 and conferred wild type as well as the wild-type sensitivity to rapamycin. Mn2+ additions to media partially suppressed rapamycin resistance of wild type and pmr1 tor1, suggesting that Tor1 and Tor2 are regulated by manganese. We parsed the roles of Ca2+ and Mn2+ transport and the compartments in rapamycin response using separation-of-function mutants available for Pmr1. A strain containing the D53A mutant (Mn2+ transporting) of Pmr1 is rapamycin sensitive, but the Q783A mutant (Ca2+ transporting) strain is rapamycin resistant. Mn2+ transport into the Golgi lumen appears to be required for rapamycin sensitivity. Overexpression of Ca2+ pump SERCA1, Ca2+/H+ antiporter Vcx1, or a Mn2+ transporting mutant of Vcx1 (Vcx1-M1) failed to restore rapamycin sensitivity, and loss of Pmr1 but not other transporters of Ca2+ or Mn2+ results in rapamycin resistance. Overexpression of Ccc1, a Fe2+ and Mn2+ transporter that has been localized to Golgi and the vacuole, does restore rapamycin sensitivity to pmr1Δ. We conclude that Mn2+ in the Golgi inhibits TORC1 signaling.
PMR1 is a member of the P-type ATPase family of ion transporters (Rudolph et al. 1989; Antebi and Fink 1992). P-type ATPases bind ATP and are phosphorylated at a step in their transport cycles (Vanoevelen et al. 2005). Pmr1 transports Ca2+ or Mn2+ ions with high affinity from the cytoplasmic space into compartments of the secretory pathway (Rudolph et al. 1989). Pmr1 helps maintain cytoplasmic Ca2+ concentration at low values that inhibit signaling to Ca2+ effectors until a stimulus, often a cellular stress, induces a transient rise in cytoplasmic Ca2+ (Cyert 2003; Mulet et al. 2006). A pmr1 mutant increases cytosolic Ca2+ and increases Ca2+ entry and accumulation in the vacuole. Pmr1 also provides compartments of the secretory pathway lumenally with Ca2+ and Mn2+. There are possible roles for both ions within the Golgi, including roles in protein modification, in regulation of sorting and vesicular traffic, and in removal of toxic levels of ions. Mannosyltransferases present in the Golgi require Mn2+ as a metal cofactor (Lisman 2004; Lobsanov et al. 2004). Crystal structures of several glycosyltransferases show Mn2+ binds to a conserved DXD motif in the catalytic site (Gastinel et al. 2001; Persson et al. 2001; Lobsanov et al. 2004). Strains lacking PMR1 have defects in N- and O-linked glycosylations (Rudolph et al. 1989; Olivero et al. 2003). The secretory pathway is also used to remove metal ions, such as Mn2+, that exceed physiologic levels. PMR1 mutants are notably sensitive to high concentrations of extracellular Mn2+ (Lapinskas et al. 1995).
Recently we reported that Saccharomyces cerevisiae strains with a pmr1 deletion have increased resistance to rapamycin (Devasahayam et al. 2006). Rapamycin is an immunosuppressive that in complex with Fpr1 (FKBP12) inhibits a subset of functions of the target of rapamycin (TOR) proteins (Crespo and Hall 2002). There are two TOR genes (TOR1 and TOR2) in S. cerevisiae (reviewed in Crespo and Hall 2002; De Virgilio and Loewith 2006) that encode large (∼280 kDa) proteins highly conserved throughout evolution that have atypical serine/threonine protein kinase activity, yet are related to phosphatidylinositol 3-kinase protein kinases. TOR2, but not TOR1, is essential in S. cerevisiae. TOR is found in two conserved complexes (TORC1 and TORC2) with distinct composition and function (Crespo and Hall 2002; De Virgilio and Loewith 2006); TORC1, but not TORC2, is inhibited by rapamycin. Deletion of TOR1 consistently causes rapamycin hypersensitivity.
TOR is a central regulator of cell growth and promotes an increase in cell size but not cell number (Edgar 2006). How TOR regulates cell growth and how nutrient signals regulate TOR are intensely studied problems (Wullschleger et al. 2006). Given the many cellular processes affected, regulation by TOR of one biological process may be paramount and effects on other cellular processes more indirect. Vesicular trafficking may be a candidate process as it is required for both isotropic and spatially restricted growth and is dependent upon actin dynamics (Neufeld 2007). Treatment with rapamycin mimics starvation for nitrogen in S. cerevisiae despite availability of nutrients, resulting in endocytosis and degradation of specific permeases and their substitution by the general amino acid permease, Gap1 (Crespo and Hall 2002). In Drosophila melanogaster, genetic studies of an eye phenotype that results from tissue-specific overexpression of TOR suggest TOR signaling suppresses endocytosis and degradation of specific proteins (Hennig et al. 2006). Overexpression of Rheb in D. melanogaster, placed in genetic models upstream of TOR, also increased cell-surface expression of Slimfast, a cationic amino acid transporter (Hennig et al. 2006). In human FL5.12 cells, survival and growth requires cell-surface expression of nutrient transporters whose stability at the membrane requires interleukin-3 (IL3) signaling (Edinger and Thompson 2004). Expression of an allele of mTOR having constitutive activity, but not wild-type mTOR, conferred IL3 independence to FL5.12 by an unidentified mechanism that stabilized nutrient transporters in the absence of IL3 (Edinger and Thompson 2004). These observations strongly indicate that regulation of nutrient transporters by TOR is conserved from yeast to man.
TOR localization is controversial (Martin et al. 2006), which may indicate the localization is dynamic and regulated. Studies in yeast and mammalian systems agree that TOR is peripherally associated with intracellular membranes. The localization of mTOR was reported as ER and Golgi (Drenan et al. 2004) and recently further supported by identification of putative targeting sequences (Liu and Zheng 2007). In S. cerevisiae, TOR1 has been localized by biochemical fractionation and immunogold electron microscopy (Martin et al. 2006). TOR1 was found in fractions with density of plasma membranes and in a lighter, unidentified pool. TOR2 has been reported to be associated with vacuolar membranes (Cardenas and Heitman 1995).
In this article we describe interactions of PMR1 and TOR-dependent signaling pathways. We parse the roles of Ca2+ and Mn2+ transport by Pmr1 in rapamycin sensitivity. We show that multiple transporters that affect Ca2+ and Mn2+ do not affect the rapamycin response, suggesting that the effect is specific to Pmr1. We use separation-of-function mutants to show that Mn2+ transport by Pmr1 suffices to restore rapamycin sensitivity. We used transporters that are specific to individual compartments to demonstrate that Mn2+ transport by Pmr1 into the Golgi is required for rapamycin sensitivity in yeast.
MATERIALS AND METHODS
Strains:
The yeast strains used in this study are listed in Table 1, except for strains in Figures 1C and 2A, which are deletion mutants from the haploid collection (replaced by KanMX4) in BY4741 background. The sod1 mutants are in EG103 background (Lapinskas et al. 1995). YGD25 (pmr1Δ tor1Δ) was made by crossing MH349-3d (tor1Δ in JK9-3da) (Helliwell et al. 1994) with LJ25-2C (pmr1Δ in TB50α), sporulated for 2–3 days on KAc plates, and tetrads were dissected. Double mutants were selected as being G418 resistant, grown on leu− plates, and were mating-type α.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | EUROSCARF |
| TB50a | MATaleu2-3, 112 ura3-52 rme1 trp1 his3Δ1 HMLa | Beck and Hall (1999) |
| YGD3 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 pmr1Δ∷KanMX4 | EUROSCARF deletion set |
| LJ25-1A | MATaleu2-3, 112 ura3-52 rme1 trp1 his3Δ1 HMLapmr1Δ∷KanMX4 | Devasahayam et al. (2006) |
| LJ25-2C | MATα leu2-3, 112 ura3-52 rme1 trp1 his3Δ1 HMLapmr1Δ∷KanMX4 | This study |
| EG103 | MATα leu2-3,112 his3Δ1 trp-289 ura3-52 Gal+ | Lapinskas et al. (1995) |
| EG133 | MATα leu2-3,112 his3Δ1 sod1Δ∷URA3 sod2Δ∷TRP1 Gal+ | Lapinskas et al. (1995) |
| PJKP-1 | MATα his3Δ1 sod1Δ∷URA3 sod2Δ∷TRP1 pmr1Δ∷LEU2 Gal+ | Lapinskas et al. (1995) |
| MH349-3d | MATaleu2-3, 112 ura3-52 rme1 trp1 his4 HMLator1Δ∷LEU2-4 | Helliwell et al. (1994) |
| YGD25 | MATα leu2-3, 112 ura3-52 rme1 trp1 HMLator1Δ∷LEU2-4 pmr1Δ∷KanMX4 | This study |
Figure 1.—
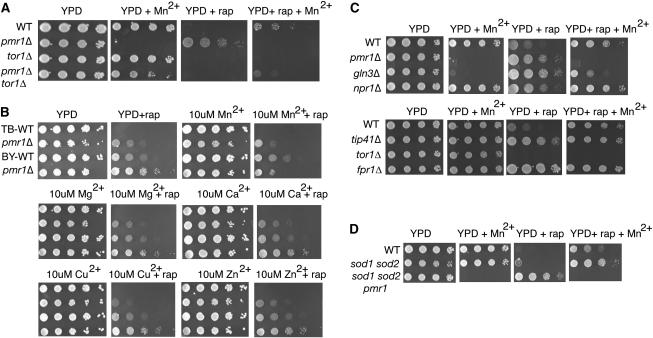
Mn2+ suppresses rapamycin resistance of pmr1Δ. (A) pmr1Δ tor1Δ grows in the presence of extracellular Mn2+; i.e., pmr1Δ is synthetically rescued by tor1Δ. tor1Δ (MH349-3d) is not rapamycin resistant (20 ng/ml) in the presence of extracellular Mn2+ (2 mm) unlike WT (TB50a) and pmr1Δ tor1Δ (YGD25). pmr1Δ tor1Δ is rapamycin sensitive as is tor1Δ and WT. Cells were grown for 3–5 days at 30°. (B) Growth of pmr1Δ strains (LJ25-1A and YGD3) is compared to their WTs (TB50a and BY4741, respectively). TB-WT (TB50a) has a genetic background more sensitive to rapamycin than BY-WT (BY4741) (EUROSCARF). Extracellular Mn2+ suppresses rapamycin (20 ng/ml) resistance of pmr1Δ (LJ25-1A and YGD3) at 10 μm. Other divalent cations (chloride salts) do not suppress rapamycin resistance of pmr1Δ at 10 μm. Cells were grown for 3 days at 30°. (C) Mutants of the TOR pathway on rapamycin (20 ng/ml) and 2 mm Mn2+. gln3Δ is Mn2+ hypersensitive and rapamycin resistant as is pmr1Δ (YGD3), but rapamycin resistant in the presence of extracellular Mn2+ (2 mm), unlike pmr1Δ. tor1Δ (BY4741) is sensitive in the presence of rapamycin and Mn2+ (2 mm). Cells were grown for 3 days at 30°. WT: BY4741. Deletion mutants are from the EUROSCARF deletion collection in BY4741 background. (D) sod1Δ sod2Δ (EG133) is rapamycin (20 ng/ml) resistant compared to WT (EG103) and Mn2+ (2 mm) enhances this phenotype. Deletion of PMR1 in sod1Δ sod2Δ (PJKP-1) also enhances the rapamycin-resistant phenotype.
Figure 2.—
Mn2+ transport into the Golgi is required for rapamycin-sensitive signaling in yeast. (A) Of the Ca2+ and Mn2+ transporters in a yeast cell, only deletion of PMR1 confers rapamycin resistance. Deletion mutants were from the EUROSCARF yeast deletion collection in BY4741; cells were grown to midlog phase, serially diluted, and spotted on YPD and YPD containing 100 ng/ml rapamycin. (B) A Pmr1p mutant impaired for Ca2+ transport rescues rapamycin sensitivity. Mn2+ transporting mutant (D53A) of Pmr1 defective for Ca2+ transport suppresses rapamycin resistance. WT and D53A, Q783A, and D778A Pmr1 mutants were expressed in pmr1Δ (YGD3) from endogenous promoter on a YCp plasmid. Cells were spotted on SC −leu and SC −leu media containing 100 ng/ml rapamycin or 2 mm MnCl2 or both.
Plasmids:
Plasmids to express pmr1 point mutations D53A, Q783A, and D778A were constructed by subcloning a fragment [D53A (300 bp XbaI), D778A (∼2.2 kb BamHI–PstI), and Q783A (∼2.2 kb BamHI–PstI] from the EBc24-2L1 mutants in pRS415 (Bolton et al. 2002) (gift of Jeff Boeke) into the same sites of YCp pHAC111-PMR1 (Devasahayam et al. 2006). All three were sequenced and contained the point mutations. Ccc1 was overexpressed using plasmid pOSC10 that contains CCC1 under control of the MET3 promoter and tagged with FLAG epitope (gift of Jerry Kaplan) (Li et al. 2001). Rabbit SERCA1a cDNA (Durr et al. 1998) (gift of Hans Rudolph) is expressed from the constitutive yeast PMA1 promoter in pRS316 (br434). VCX1 and VCX1-M1 cDNA were expressed from high-copy yeast-expression shuttle vector p2UGpd (Pittman et al. 2004), containing the strong constitutive GPD promoter. CAX1 and CAX2 were cloned into yeast shuttle vector piHGpd for expression in yeast.
Media:
Rapamycin (Sigma, St. Louis) was in 90% ethanol/10% Tween-20. 1, 2-bis (2-aminophenoxy) ethane-N, N, N′, N′-tetraacetic acid (BAPTA) and ethylene glycol-bis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA)-containing media were prepared as described and pH adjusted to 6.0 (with 1 n KOH) and 5.5 (0.5 m MES buffer), respectively (Durr et al. 1998; Wei et al. 2000). Mn2+ plates contained MnCl2; Ca2+ plates, CaCl2. For spottings, cells were 10-fold serially diluted from an initial OD600 of 1.0. Yeast transformations were done and standard yeast media prepared as described (Amberg et al. 2005).
Western blotting:
CCC1 expression was induced in pmr1Δ (LJ25-1A/a) in the absence of methionine and detected by immunoblot with mouse anti-FLAG M2 antibody (Sigma) at 0.5 μg/ml. Cells were grown in 5 ml SC −leu or SC −leu met medium and protein was extracted by the alkali lysis method (Kushnirov 2000). Lysis buffers contain 350 μg/ml p-methyl sulfonyl fluoride, 1×. Complete protease inhibitor (Roche, Indianapolis) and 1 μm phosphatase inhibitor (microcystin-LR).
RESULTS
tor1Δ suppresses the manganese sensitivity of pmr1Δ:
Pmr1 is a negative regulator of TORC1 signaling (Devasahayam et al. 2006). Since loss of TOR1 causes rapamycin hypersensitivity (T. F. Chan et al. 2000), we looked for synthetic interactions of tor1Δ with pmr1Δ. Throughout this article, we infer TORC1 signaling from how cells respond to the drug. Reduction of TORC1 signaling results in rapamycin resistance and enhanced TORC1 signaling results in rapamycin sensitivity. Strains with a pmr1 mutation do not grow on media containing millimolar concentrations of the divalent cation Mn2+ (Lapinskas et al. 1995). The Mn2+ sensitivity of pmr1Δ cells was completely suppressed by deleting TOR1 (Figure 1A), suggesting Mn2+ sensitivity of Pmr1 was due to hyperactive Tor1. In addition, tor1Δ pmr1Δ was sensitive to rapamycin unlike pmr1Δ. Thus, deletion of TOR1 restored the wild-type phenotype in each case. Namely, loss of TOR1 restored the ability of the pmr1Δ strain to grow on Mn2+-containing media such as WT, and loss of TOR1 restored the rapamycin sensitivity of the pmr1Δ strain to wild-type sensitivity. Interestingly, rapamycin sensitivity of wild type (TB50a) and tor1Δ pmr1Δ was partially suppressed in media containing 2 mm Mn2+ (Figure 1A).
These data are consistent with a proposed genetic model in which Pmr1 is a negative regulator of TORC1 signaling (Devasahayam et al. 2006) and suggest that the mechanism may be through regulating manganese homeostasis. Intracellular Mn2+ increases dramatically in pmr1Δ because Mn2+ fails to enter the secretory pathway, whereby excess Mn2+ can be removed (Culotta et al. 2005). Since TOR1 is required for Mn2+ toxicity in pmr1Δ, TORC1 appears toxic in this circumstance. Smaller increases in TORC1 activity by Mn2+, in the absence of pmr1Δ or in the presence of pmr1Δ with tor1Δ, may allow weak growth in the presence of rapamycin.
Extracellular Mn2+ increases rapamycin resistance:
TOR proteins are members of the phosphatidylinositol 3-kinase-related protein kinases that include ataxia-telangiectasia mutated (ATM) (Wullschleger et al. 2006). Both the TOR proteins (Alarcon et al. 1999) and ATM (D. W. Chan et al. 2000) have been reported to require Mn2+ as a cofactor with ATP for maximal activity. Mn2+ entry into cells is suppressed by feedback inhibition of expression of the Mn2+ transporter Smf1p, but high concentrations of Mn2+ still favor entry by alternative, less efficient mechanisms allowing Mn2+ to reach toxic levels in pmr1Δ (Culotta et al. 2005).
To distinguish whether low or high extracellular Mn2+ was required to increase rapamycin resistance, we studied the dose response for Mn2+ in two genetic backgrounds, BY4741 and TB50a (Figure 1B). TB50a is more rapamycin sensitive than BY4741, possibly due to the greater number of auxotrophies for amino acids. The highest concentration of Mn2+ tested, 250 μm, increased growth of wild-type strains TB50a and BY4741 on rapamycin but the lower micromolar concentrations of Mn2+ did not (data not shown). The toxic effect of Mn2+ on growth of the pmr1Δ strain was detectable at 10 μm. Rapamycin resistance of pmr1Δ is suppressed by 10 μm Mn2+, in both strain backgrounds. To rule out that this effect may be due to osmolarity, we tested the effect of other divalent cations, Ca2+, Mg2+, Cu2+, and Zn2+, at the same concentration on pmr1 cells. Only Mn2+ suppressed the rapamycin resistance of pmr1 cells (Figure 1B). Mn2+ can replace Ca2+ in media for growth in some circumstances (Loukin and Kung 1995). Additions of Ca2+ to media (1–10 mm) did not increase rapamycin resistance of BY4741 or TB50a (supplemental data at http://www.genetics.org/supplemental/).
The increase in rapamycin resistance by extracellular Mn2+ requires GLN3:
Yeast treated with rapamycin act as if starved of nitrogen despite the presence of a good nitrogen source in media. Cells respond to rapamycin by inducing transcription of genes for use of alternative nitrogen sources and by expressing active permeases such as Gap1 to scavenge nitrogen. Gln3, Npr1, and Tip41 positively regulate Gap1 in response to poor nitrogen sources (Magasanik and Kaiser 2002), and their activities are inhibited by TORC1 signaling. Deletions of these genes confer rapamycin resistance. Nuclear Gln3 transactivates genes including GAP1 that enable adaptation to poor nitrogen sources. A serine/threonine protein kinase, Npr1, positively regulates Gap1, and negatively regulates the more specific amino acid permeases, by control of their sorting and stability (De Craene et al. 2001). Nuclear translocation of Gln3 requires Sit4 phosphatase (Beck and Hall 1999). Tip41 is a Sit4 regulator, and its loss prevents Sit4-dependent dephosphorylation and activation of Npr1 (Jacinto et al. 2001). We compared the effect of Mn2+ on the wild-type BY4741 strain of S. cerevisiae and otherwise isogenic strains with deletions in these genes (Figure 1C).
All of these strains grew normally on media containing 2 mm Mn2+ with the exception of pmr1Δ and gln3Δ. Surprisingly, both the pmr1Δ strain and the gln3Δ strain were very sensitive to Mn2+. On media containing rapamycin, loss of PMR1 induced resistance to a similar degree as loss of NPR1, GLN3, or TIP41. [Deletion of FPR1 (FKBP12) serves as benchmark for rapamycin resistance.] On media additionally containing Mn2+, rapamycin resistance was increased for the wild-type BY4741 strain, confirming the observation (Figure 1A) with TB50a. The suppression of rapamycin sensitivity of BY471 by Mn2+ (compare YPD + Rap + Mn2+ to YPD + Rap) was reduced by gln3Δ but not by npr1Δ or tip41Δ. Moreover, the growth defect of gln3Δ on Mn2+ media is partially TORC1 dependent because rapamycin decreases the Mn2+ sensitivity of gln3Δ (compare YPD + Rap + Mn2+ to YPD + Mn2+). Extracellular Mn2+ was unable to suppress rapamycin hypersensitivity of tor1Δ.
A pmr1 mutant is a suppressor of the aerobic growth defect that occurs in a strain lacking Sod1 (Lapinskas et al. 1995), a Cu2+ containing superoxide dismutase localizing to the cytoplasmic compartment. Suppression is due to an increase of cellular Mn2+ to scavenge reactive oxygen species (Sanchez et al. 2005). A large screen identified sod1 from the genome-deletion set as having rapamycin resistance (Xie et al. 2005). We tested a sod1 sod2 strain (gift of Valerie Culotta), lacking both cytoplasmic (Sod1) and mitochondrial superoxide dismutase (Sod2), for rapamycin resistance (Figure 1D). Indeed, the sod1Δ sod2Δ strain was slightly more resistant to rapamycin in comparison to its wild type (EG103). Either extracellular Mn2+ or deletion of PMR1 suppressed the growth defect of sod1 sod2 on rapamycin. This result implicates a reciprocal role of reactive oxygen species (ROS) and Mn2+ in TORC1 signaling.
Pmr1-specific Mn2+ transport into Golgi is essential for TORC1 signaling:
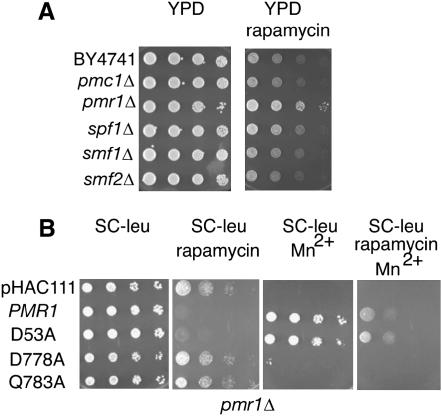
Pmr1 affects both calcium and manganese homeostasis (Rudolph et al. 1989; Antebi and Fink 1992). To determine which divalent cation is responsible for the rapamycin responses we compared the pmr1Δ strain to strains deleted for other cation transporters. Cells from mutants lacking the vacuolar Ca2+ transporter gene PMC1 (Cunningham and Fink 1994), the plasma membrane Mn2+ transporter SMF1 (Liu and Culotta 1999), the vesicular Mn2+ transporter SMF2 (Portnoy et al. 2000), or the ER-localized P-type ATPase SPF1 (Cronin et al. 2002) all had the same rapamycin sensitivity as a wild-type-cells strain (Figure 2A). Interestingly, only pmr1Δ conferred rapamycin resistance. The P-type ATPase Spf1 is involved in Ca2+ homeostasis (Cronin et al. 2002), but did not affect rapamycin sensitivity, suggesting that manganese is the important cation that modulates the response to rapamycin.
Separation-of-function Pmr1 mutants implicate Mn2+ in TORC1 signaling:
Point mutations of PMR1 that confer selective transport for Ca2+ or Mn2+ have been described (Wei et al. 1999, 2000; Mandal et al. 2000). The mutations were identified by screening for suppression of Ca2+ or Mn2+ phenotypes in pmr1 pmc1 cnb1 cells. The D53A mutant rescued the Mn2+ phenotype and Q783A rescued the Ca2+ phenotype, and both Q783A and D53A Pmr1 were localized to Golgi (Wei et al. 2000). D778A is nonfunctional and serves as an additional control. We compared mutants D53A and Q783A to determine if preferential loss of Ca2+ or Mn2+ transport into Golgi was more important for the rapamycin resistance of pmr1Δ (Figure 2B). The pmr1D53A cells (defective for Ca2+ transport) were rapamycin sensitive, whereas the strains transformed with pmr1Q783A cells (defective for Mn2+ transport) and D778A (nonfunctional) mutants were rapamycin resistant. D53A transports Mn2+ normally, and it suppressed Mn2+ toxicity as did wild-type Pmr1 (Figure 2B). To confirm that pmr1Q783A was functional, we determined that it suppressed BAPTA sensitivity, the Ca2+ phenotype (supplemental data at http://www.genetics.org/supplemental/).
Manganese in the Golgi modulates TOR activity:
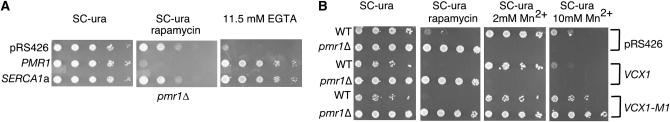
We used transporters that are specific to individual compartments to demonstrate that Mn2+ transport by Pmr1 into the Golgi is required for rapamycin sensitivity in yeast. To rule out the lack of transport of cytosolic Ca2+ into the secretory pathway as the cause of rapamycin resistance, we also studied pmr1Δ cells expressing mammalian SERCA1. Notably, SERCA1 did not complement the rapamycin resistance of pmr1Δ (Figure 3A). This P2-type Ca2+ ATPase restores growth of a pmr1Δ strain in EGTA- or BAPTA-containing media (Durr et al. 1998; Degand et al. 1999). In contrast, SERCA1 was unable to restore growth of pmr1Δ in high Mn2+ (Ton and Rao 2004). This suggests rapamycin resistance of pmr1Δ is not due to entry of Ca2+ into the secretory pathway.
Figure 3.—
Ca2+ transport into the secretory pathway is not required for rapamycin sensitivity (A) Normalizing intracellular Ca2+ with SERCA1a does not suppress rapamycin resistance of pmr1Δ. YGD3 was transformed with PMR1 (pKC21, gift of K. Cunningham) or SERCA1a expressed on a YCp vector with yeast PMA1 promoter (br434) or control plasmid (Durr et al. 1998) (gift of H. Rudolph). Cells were serially diluted and spotted; media were as labeled. (B) Vacuolar Mn2+ or Ca2+ transport does not suppress rapamycin phenotype of pmr1Δ. VCX1-M1, Mn2+ transporting mutant of vacuolar H+/Ca2+ antiporter VCX1, suppresses Mn2+ hypersensitivity of pmr1Δ (YGD3) yet does not restore rapamycin sensitivity. Overexpression of VCX1 in pmr1Δ also does not restore rapamycin sensitivity; rapamycin, 20 ng/ml; MnCl2, 2 mm. Cells were fivefold serially diluted from a starting OD600 of 2.0. Plates were incubated for 5 days at 30°. WT: BY4741.
VCX1 encodes the Ca2+/H+ exchanger in the vacuolar membrane (Pittman et al. 2004). An L208P mutant (VCX1-M1) has enhanced Mn2+/H+ exchange, and this mutant suppresses the Mn2+ toxicity phenotype of pmr1Δ much better than Vcx1p (Pittman et al. 2004). High-dosage expression of neither Vcx1p nor the Mn2+ selective mutant L208P suppressed rapamycin resistance due to loss of Pmr1 (Figure 3B). This result suggests that lowering cytosolic Ca2+ or Mn2+ by transport to the vacuolar space does not restore rapamycin sensitivity of pmr1Δ. The Arabadopsis thaliana CAX1 and CAX2 genes encode Ca2+/H+ exchangers that localize to the yeast vacuole (Shigaki et al. 2003). Cax2 transports Mn2+ better than Cax1, and expression of Cax1 and Cax2 decreases the Mn2+ toxicity phenotype of pmr1Δ (Shigaki et al. 2003). Neither Cax1 nor Cax2 restored rapamycin sensitivity of pmr1 (supplemental data at http://www.genetics.org/supplemental/), confirming that lowering Ca2+ or Mn2+ in the cytoplasmic compartment by transport into the vacuole was not sufficient to restore rapamycin sensitivity. Together these data support our conclusion that cytosolic Mn2+ does not regulate TOR activity and suggest that the regulation is achieved in a different cellular compartment.
CCC1 restores rapamycin sensitivity:
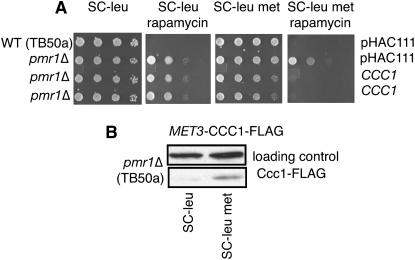
Pmr1 has been localized to the Golgi (Rudolph et al. 1989; Antebi and Fink 1992). To determine if the manganese-dependent regulation of TORC1 signaling was dependent on the Golgi, we analyzed the effect of overexpressing CCC1 in pmr1Δ cells. CCC1 was isolated as a high-dosage suppressor of Mn2+ sensitivity of a cnb1 strain (Pozos et al. 1996) and was isolated in an independent screen as a suppressor of the marked Mn2+ sensitivity of pmr1 (Lapinskas et al. 1996). Furthermore, Ccc1 has been reported to localize to the Golgi (Lapinskas et al. 1996). We asked if CCC1 could complement rapamycin resistance of pmr1Δ (Figure 4). Excess expression of Ccc1p from the inducible MET3 promoter in the pmr1Δ strain restored wild-type sensitivity to rapamycin, fully complementing pmr1Δ for this phenotype. We conclude that Mn2+ in the Golgi inhibits TORC1 signaling.
Figure 4.—
Interactions of PMR1 with genes involved in Mn2+ homeostasis. (A) Ccc1, a putative Fe2+/Mn2+ transporter (Li et al. 2001), suppresses the rapamycin-resistant phenotype of pmr1Δ (LJ25-1A) in SC −leu, met medium containing 20 ng/ml rapamycin. Cells were 10-fold serially diluted from a starting OD600 of 5.0. (B) CCC1 expression from the pMET3-CCC1-FLAG plasmid (pOSC10) is induced in SC −leu met medium (in the absence of methionine) but not in SC −leu medium in the LJ25-1A strain background. Mouse anti-FLAG antibody detects Ccc1 tagged with FLAG epitope.
DISCUSSION
In this study we show that TOR1 is required for Mn2+ toxicity of pmr1Δ, consistent with our model that Pmr1 is a negative regulator of TORC1 signaling (Devasahayam et al. 2006). Extracellular Mn2+ also improved growth of wild-type and pmr1Δ tor1Δ cells. Since some phosphatidylinositol 3-kinase-related protein kinases are Mn2+ dependent, this suggested that an increase in cytosolic Mn2+ increases Tor1 activity, bypassing rapamycin inhibition. Experiments to parse Ca2+ and Mn2+ in rapamycin resistance and define the compartments in which Ca2+ or Mn2+ were important led to the following conclusions:
Loss of Mn2+ transport appears more important than loss of Ca2+ transport for rapamycin resistance in a pmr1 strain.
Restoration of rapamycin sensitivity requires Mn2+ transport into the Golgi or into a vesicular compartment in communication with Golgi.
Vacuolar Vcx1p-M1 sufficed to suppress Mn2+ sensitivity of pmr1Δ but failed to restore rapamycin sensitivity, disfavoring our initial hypothesis that an increase in cytosolic Mn2+ bypasses rapamycin to activate Tor1. The new hypothesis is that an increase in Golgi Mn2+ inhibits TORC1 signaling.
There were two surprising observations. First, loss of TOR1 suppresses the Mn2+ sensitivity of pmr1Δ. Since PMR1 is placed by epistasis upstream of TORC1 signaling (Devasahayam et al. 2006), the Mn2+ sensitivity of pmr1Δ can be rationalized as Mn2+ hyperactivity of Tor1 occurring in this context. Extracellular Mn2+ increased rapamycin resistance of pmr1 tor1. Together the data suggest Mn2+ regulates both Tor1 and Tor2 in yeast. Whether it is direct or indirect will have to be determined. Second, loss of GLN3 made cells sensitive to Mn2+, and sensitivity was reduced by rapamycin showing that the Mn2+ sensitivity of gln3Δ is in part TORC1 dependent. GLN3 target genes include a number of ion transporters in addition to the set of genes for scavenging nitrogen. ENA1, encoding a P-type ATPase of the plasma membrane that transports monovalent cations (Na+ and Li+), is a Gln3 target and its transcription is induced by rapamycin (Crespo et al. 2001). PMR1 may also be a Gln3 target gene, since gln3Δ has a pmr1Δ phenotype of Mn2+ hypersensitivity.
Ccc1p is a putative transporter for Fe2+ and Mn2+ (Li et al. 2001). Ccc1 has significant similarity to archaebacteria proteins (NP_393546, YP_023568, and NP_110542) including a DMIYGISDGL motif similar to DLIIGLSDGL in Ccc1 that could coordinate a metal ion. Ccc1p is implicated in Fe2+ ion homeostasis from the literature but not as a Ca2+ transporter. Low cytosolic Fe2+ represses CCC1 transcription and negatively regulates CCC1 mRNA by inducing CTH2 (Puig et al. 2005). High iron in media inhibits growth of a ccc1Δ strain, and high-dosage expression of CCC1 is protective of iron toxicity (Chen and Kaplan 2000). High-dosage expression of Ccc1p-FLAG can increase iron and manganese content in a purified vacuolar fraction, and Ccc1p-FLAG is concentrated at the periphery of the vacuole (Li et al. 2001). Ccc1p has been localized to the Golgi as well (Lapinskas et al. 1996), and the latter is important for our interpretation. Both localizations are likely correct, and trafficking of Ccc1p between the Golgi and the vacuole is a distinct possibility. Specific factors may determine which localization predominates. Even if Ccc1 is in the vacuole, it does not preclude it from functioning in the Golgi. Ellis et al. show vacuolar zinc transporters functioning in the ER (Ellis et al. 2004). Transporters may have activity to transport metals into the lumen of the secretory pathway as they are being trafficked to their final destination.
There are two good ways to imagine how Mn2+ in the Golgi regulates rapamycin sensitivity in yeast. The first is that Mn2+ regulates permease routing or function. One candidate from what we know from the literature is mannosylation reactions of proteins or lipids (Lisman 2004). Glycosylations may affect sorting and plasma membrane localization of specific proteins (Proszynski et al. 2004). Mannosylation of proteins requires Mn2+, and this suggests that mannosylation of lipids, as with proteins, could require Mn2+. Indeed, sphingolipid mannosylation in yeast requires Csg2-dependent Mn2+ transport into the lumen of early secretory organelles, and conversion of inositol phosphoryl-ceramide (IPC) to mannosylinositol phosphoceramide in a csg2 strain is partially suppressed by the addition of millimolar Mn2+ to media (Lisman 2004). IPC is synthesized in the medial Golgi from ceramide precursors made in the ER. Sphingolipids play an important role in sorting proteins, including nutrient permeases, destined for the plasma membrane via formation of detergent-resistant membrane microdomains (Proszynski et al. 2005). The second possibility is that some TOR may be localized to the Golgi, as suggested for mammalian Tor (Liu and Zheng 2007), where there is access to Mn2+ within the secretory pathway. Further work is necessary to define how Mn2+ is involved in sensitivity to rapamycin in yeast.
Acknowledgments
We thank Jerry Kaplan for discussions and for suggesting our CCC1 experiment, as well as Valerie Culotta, Robbie Loewith, Kendal Hirschi, Jeff Boeke, and Rajini Rao for reagents and/or advice. Michael Hall made helpful suggestions for structuring the manuscript. Hans Rudolph suggested the Mn2+ supplement experiment. Support from the Department of Pharmacology is acknowledged. This work was supported by National Institutes of Health grants GM62890 (T.W.S.) and GM40334 (D.J.B.).
References
- Alarcon, C. M., J. Heitman and M. E. Cardenas, 1999. Protein kinase activity and identification of a toxic effector domain of the target of rapamycin TOR proteins in yeast. Mol. Biol. Cell 10: 2531–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg, D., D. J. Burke and J. N. Strathern, 2005. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Antebi, A., and G. R. Fink, 1992. The yeast Ca (2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 3: 633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, T., and M. N. Hall, 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402: 689–692. [DOI] [PubMed] [Google Scholar]
- Bolton, E. C., A. S. Mildvan and J. D. Boke, 2002. Inhibition of reverse transcription in vivo by elevated manganese ion concentration. Mol. Cell 9: 879–889. [DOI] [PubMed] [Google Scholar]
- Cardenas, M. E., and J. Heitman, 1995. FKBP12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4 kinase activity. EMBO J. 14: 5892–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, D. W., S. C. Son, W. Block, R. Ye, K. K. Khanna et al., 2000. Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase. J. Biol. Chem. 275: 7803–7810. [DOI] [PubMed] [Google Scholar]
- Chan, T. F., J. Carvalho, L. Riles and X. F. Zheng, 2000. A chemical genomics approach toward understanding the global functions of the target of rapamycin protein (TOR). Proc. Natl. Acad. Sci. USA 97: 13227–13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, O. S., and J. Kaplan, 2000. CCC1 suppresses mitochondrial damage in the yeast model of Friedreich's ataxia by limiting mitochondrial iron accumulation. J. Biol. Chem. 275: 7626–7632. [DOI] [PubMed] [Google Scholar]
- Crespo, J. L., and M. N. Hall, 2002. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 66: 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo, J. L., K. Daicho, T. Ushimaru and M. N. Hall, 2001. The GATA transcription factors GLN3 and GAT1 link TOR to salt stress in Saccharomyces cerevisiae. J. Biol. Chem. 276: 34441–34444. [DOI] [PubMed] [Google Scholar]
- Cronin, S. R., R. Rao and R. Y. Hampton, 2002. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 157: 1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta, V. C., M. Yang and M. D. Hall, 2005. Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot. Cell 4: 1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, K. W., and G. R. Fink, 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 124: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert, M. S., 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311: 1143–1150. [DOI] [PubMed] [Google Scholar]
- De Craene, J. O., O. Soetens and B. Andre, 2001. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 276: 43939–43948. [DOI] [PubMed] [Google Scholar]
- De Virgilio, C., and R. Loewith, 2006. Cell growth control: little eukaryotes make big contributions. Oncogene 25: 6392–6415. [DOI] [PubMed] [Google Scholar]
- Degand, I., P. Catty, E. Talla, D. Thines-Sempoux, A. de Kerchove d'Exaerde et al., 1999. Rabbit sarcoplasmic reticulum Ca (2+)-ATPase replaces yeast PMC1 and PMR1 Ca (2+)-ATPases for cell viability and calcineurin-dependent regulation of calcium tolerance. Mol. Microbiol. 31: 545–556. [DOI] [PubMed] [Google Scholar]
- Devasahayam, G., D. Ritz, S. B. Helliwell, D. J. Burke and T. W. Sturgill, 2006. Pmr1, a Golgi Ca2+/Mn2+-ATPase, is a regulator of the target of rapamycin (TOR) signaling pathway in yeast. Proc. Natl. Acad. Sci. USA 103: 17840–17845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan, R. M., X. Liu, P. G. Bertram and X. F. Zheng, 2004. FKBP12-rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J. Biol. Chem. 279: 772–778. [DOI] [PubMed] [Google Scholar]
- Durr, G., J. Strayle, R. Plemper, S. Elbs, S. K. Klee et al., 1998. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 9: 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., 2006. How flies get their size: genetics meets physiology. Nat. Rev. Genet. 7: 907–916. [DOI] [PubMed] [Google Scholar]
- Edinger, A. L., and C. B. Thompson, 2004. An activated mTOR mutant supports growth factor-independent, nutrient-dependent cell survival. Oncogene 23: 5654–5663. [DOI] [PubMed] [Google Scholar]
- Ellis, C. D., F. Wang, C. W. MacDiarmid, S. Clark, T. Lyons et al., 2004. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J. Cell Biol. 166: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastinel, L. N., C. Bignon, A. K. Misra, O. Hindsgaul, J. H. Shaper et al., 2001. Bovine alpha1,3-galactosyltransferase catalytic domain structure and its relationship with ABO histo-blood group and glycosphingolipid glycosyltransferases. EMBO J. 20: 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell, S. B., P. Wagner, J. Kunz,M. Deuter-Reinhard, R. Henriquez et. al., 1994. TOR1 and TOR2 are structurally and functionally similar but not identical phosphatidylinositol kinase homologues in yeast. Mol. Biol. Cell 5(1): 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig, K. M., J. Colombani and T. P. Neufeld, 2006. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J. Cell Biol. 173: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto, E., B. Guo, K. T. Arndt, T. Schmelzle and M. N. Hall, 2001. TIP41 interacts with TAP42 and negatively regulates the TOR signaling pathway. Mol. Cell 8: 1017–1026. [DOI] [PubMed] [Google Scholar]
- Kushnirov, V. V., 2000. Rapid and reliable protein extraction from yeast. Yeast 16(9): 857–860. [DOI] [PubMed] [Google Scholar]
- Lapinskas, P. J., K. W. Cunningham, X. F. Liu, G. R. Fink and V. C. Culotta, 1995. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 15: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinskas, P. J., S. J. Lin and V. C. Culotta, 1996. The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol. Microbiol. 21: 519–528. [DOI] [PubMed] [Google Scholar]
- Li, L., O. S. Chen, D. McVey Ward and J. Kaplan, 2001. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276: 29515–29519. [DOI] [PubMed] [Google Scholar]
- Lisman, Q., 2004. The Golgi: a transition point in membrane lipid composition and topology. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands.
- Liu, X., and X. F. Zheng, 2007. ER and Golgi localization sequences for mammalian target of rapamycin (mTOR). Mol. Biol. Cell 18: 1073–1082. [DOI] [PMC free article] [PubMed]
- Liu, X. F., and V. C. Culotta, 1999. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J. Biol. Chem. 274: 4863–4868. [DOI] [PubMed] [Google Scholar]
- Lobsanov, Y. D., P. A. Romero, B. Sleno, B. Yu, P. Yip et al., 2004. Structure of Kre2p/Mnt1p: a yeast alpha1,2-mannosyltransferase involved in mannoprotein biosynthesis. J. Biol. Chem. 279: 17921–17931. [DOI] [PubMed] [Google Scholar]
- Loukin, S., and C. Kung, 1995. Manganese effectively supports yeast cell-cycle progression in place of calcium. J. Cell Biol. 131: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik, B., and C. A. Kaiser, 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene 290: 1–18. [DOI] [PubMed] [Google Scholar]
- Mandal, D., T. B. Woolf and R. Rao, 2000. Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 275: 23933–23938. [DOI] [PubMed] [Google Scholar]
- Martin, D. E., T. Powers and M. N. Hall, 2006. Regulation of ribosome biogenesis: Where is TOR? Cell Metab. 4: 259–260. [DOI] [PubMed] [Google Scholar]
- Mulet, J. M., D. E. Martin, R. Loewith and M. N. Hall, 2006. Mutual antagonism of target of rapamycin and calcineurin signaling. J. Biol. Chem. 281: 33000–33007. [DOI] [PubMed] [Google Scholar]
- Neufeld, T. P., 2007. TOR regulation: sorting out the answers. Cell Metab. 5: 3–5. [DOI] [PubMed] [Google Scholar]
- Olivero, I., I. Corbacho and L. M. Hernandez, 2003. The ldb1 mutant of Saccharomyces cerevisiae is defective in Pmr1p, the yeast secretory pathway/Golgi Ca(2+)/Mn(2+)-ATPase. FEMS Microbiol. Lett. 219: 137–142. [DOI] [PubMed] [Google Scholar]
- Persson, K., H. D. Ly, M. Dieckelmann, W. W. Wakarchuk, S. G. Withers et al., 2001. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs. Nat. Struct. Biol. 8: 166–175. [DOI] [PubMed] [Google Scholar]
- Pittman, J. K., N. H. Cheng, T. Shigaki, M. Kunta and K. D. Hirschi, 2004. Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol. Microbiol. 54: 1104–1116. [DOI] [PubMed] [Google Scholar]
- Portnoy, M. E., X. F. Liu and V. C. Culotta, 2000. Saccharomyces cerevisiae expresses three functionally distinct homologues of the nramp family of metal transporters. Mol. Cell. Biol. 20: 7893–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozos, T. C., I. Sekler and M. S. Cyert, 1996. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 16: 3730–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszynski, T. J., R. W. Klemm, M. Gravert, P. P. Hsu, Y. Gloor et al., 2005. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc. Natl. Acad. Sci. USA 102: 17981–17986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proszynski, T. J., K. Simons and M. Bagnat, 2004. O-glycosylation as a sorting determinant for cell surface delivery in yeast. Mol. Biol. Cell 15: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, S., E. Askeland and D. J. Thiele, 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110. [DOI] [PubMed] [Google Scholar]
- Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman et al., 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58: 133–145. [DOI] [PubMed] [Google Scholar]
- Sanchez, R. J., C. Srinivasan, W. H. Munroe, M. A. Wallace, J. Martins et al., 2005. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J. Biol. Inorg. Chem. 10: 913–923. [DOI] [PubMed] [Google Scholar]
- Shigaki, T., J. K. Pittman and K. D. Hirschi, 2003. Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J. Biol. Chem. 278: 6610–6617. [DOI] [PubMed] [Google Scholar]
- Ton, V. K., and R. Rao, 2004. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am. J. Physiol. Cell Physiol. 287: C580–C589. [DOI] [PubMed] [Google Scholar]
- Vanoevelen, J., L. Dode, K. Van Baelen, R. J. Fairclough, L. Missiaen et al., 2005. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 280: 22800–22808. [DOI] [PubMed] [Google Scholar]
- Wei, Y., V. Marchi, R. Wang and R. Rao, 1999. An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca(2+)/Mn(2+)-ATPase. Biochemistry 38: 14534–14541. [DOI] [PubMed] [Google Scholar]
- Wei, Y., J. Chen, G. Rosas, D. A. Tompkins, P. A. Holt et al., 2000. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem. 275: 23927–23932. [DOI] [PubMed] [Google Scholar]
- Wullschleger, S., R. Loewith and M. N. Hall, 2006. TOR signaling in growth and metabolism. Cell 124: 471–484. [DOI] [PubMed] [Google Scholar]
- Xie, M. W., F. Jin, H. Hwang, S. Hwang, V. Anand et al., 2005. Insights into TOR function and rapamycin response: chemical genomic profiling by using a high-density cell array method. Proc. Natl. Acad. Sci. USA 102: 7215–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]