Abstract
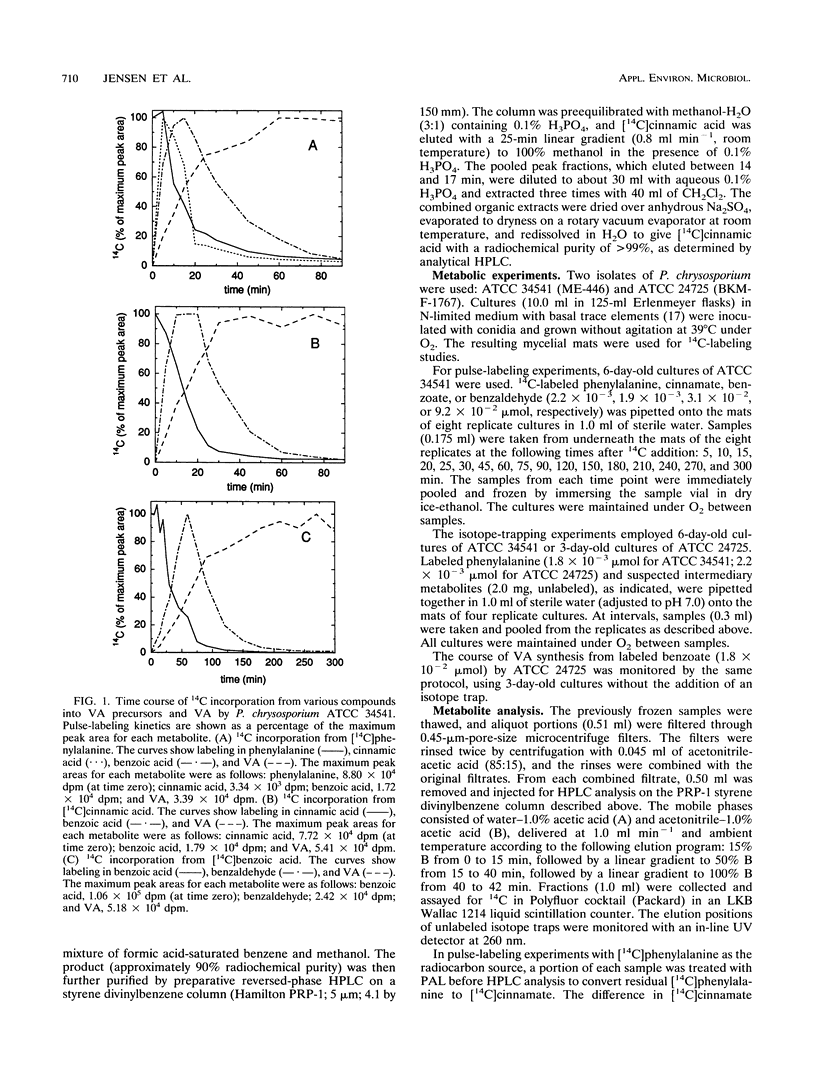
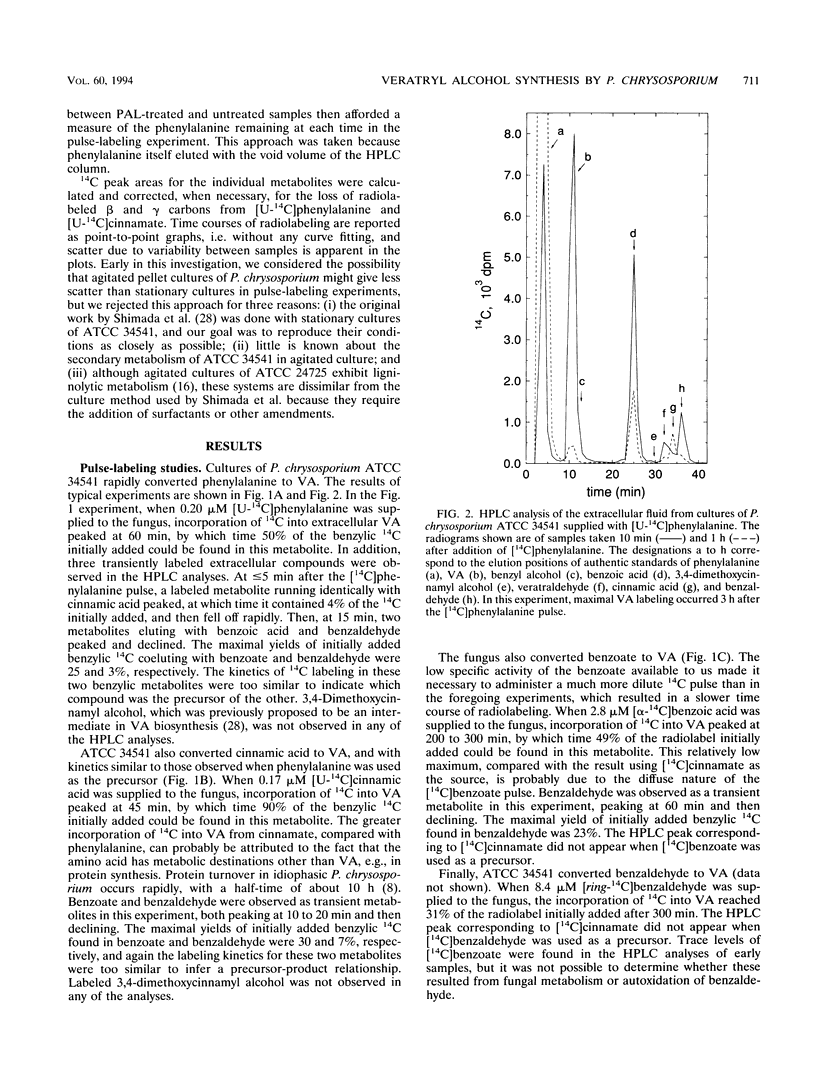
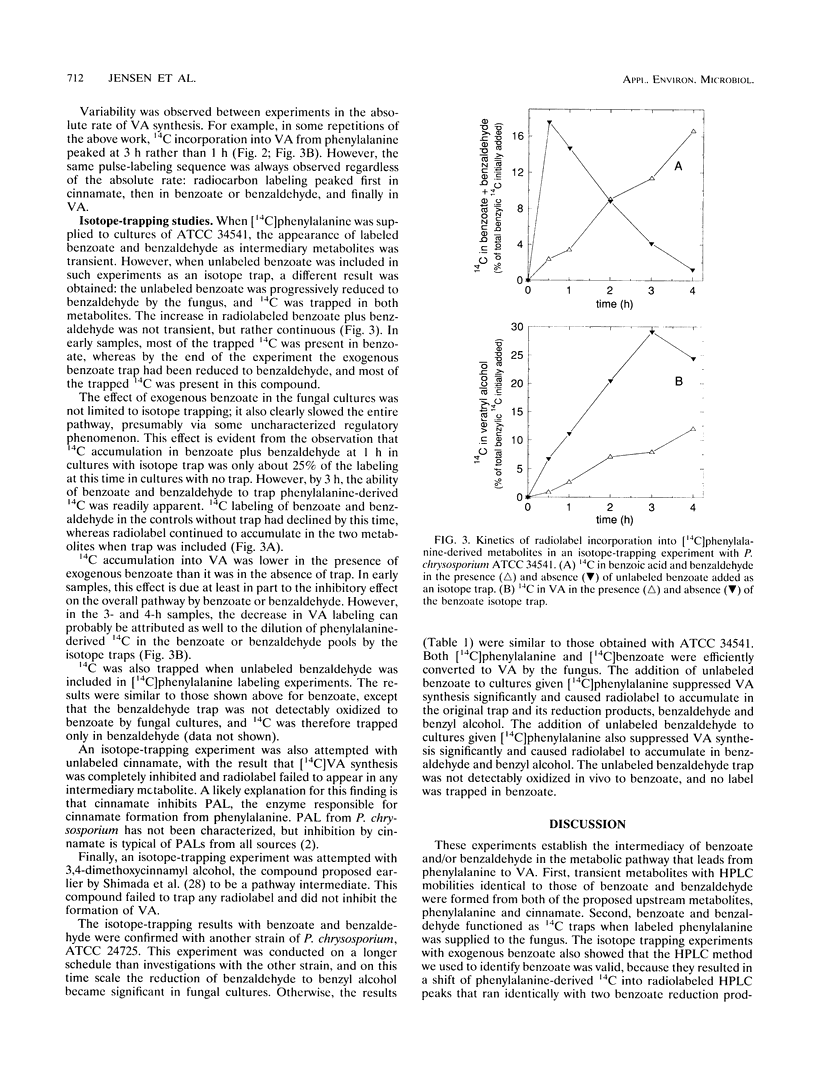
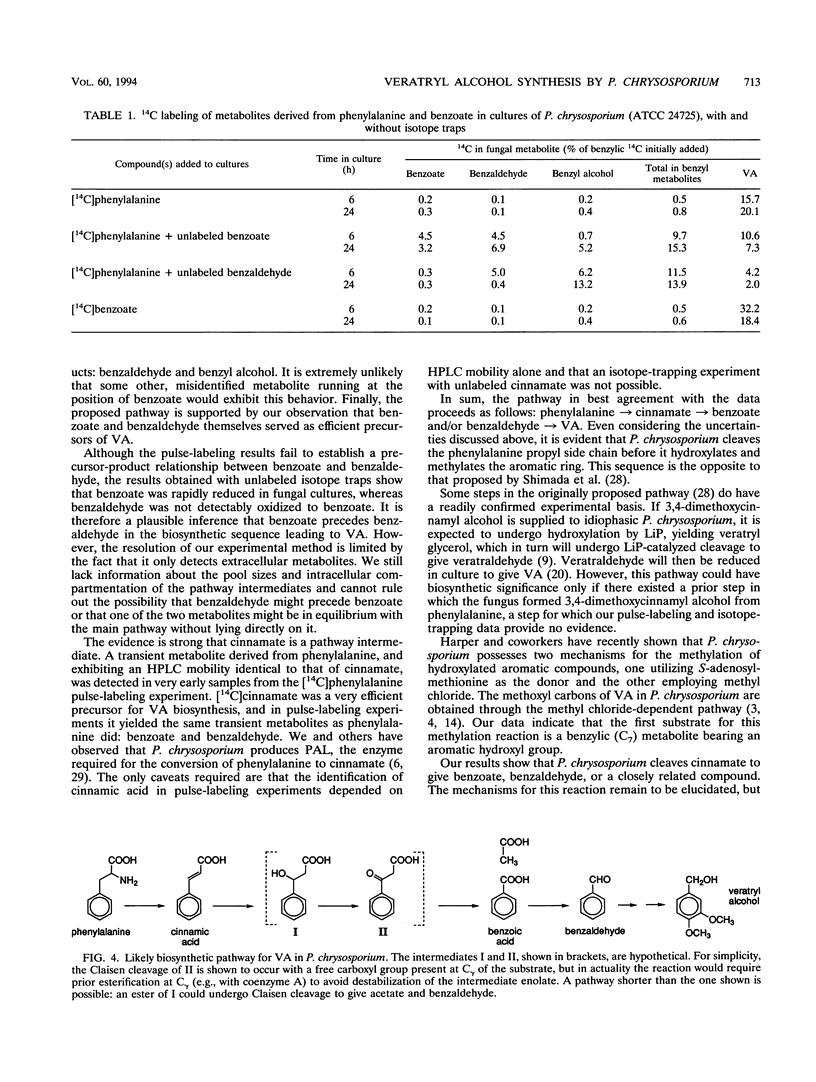
Veratryl alcohol (VA) is a secondary metabolite of white-rot fungi that produce the ligninolytic enzyme lignin peroxidase. VA stabilizes lignin peroxidase, promotes the ability of this enzyme to oxidize a variety of physiological substrates, and is accordingly thought to play a significant role in fungal ligninolysis. Pulse-labeling and isotope-trapping experiments have now clarified the pathway for VA biosynthesis in the white-rot basidiomycete Phanerochaete chrysosporium. The pulse-labeling data, obtained with 14C-labeled phenylalanine, cinnamic acid, benzoic acid, and benzaldehyde, showed that radiocarbon labeling followed a reproducible sequence: it peaked first in cinnamate, then in benzoate and benzaldehyde, and finally in VA. Phenylalanine, cinnamate, benzoate, and benzaldehyde were all efficient precursors of VA in vivo. The isotope-trapping experiments showed that exogenous, unlabeled benzoate and benzaldehyde were effective traps of phenylalanine-derived 14C. These results support a pathway in which VA biosynthesis proceeds as follows: phenylalanine → cinnamate → benzoate and/or benzaldehyde → VA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu Y., Ma D. B., Higuchi T., Shimada M. A novel enzymatic decarboxylation of oxalic acid by the lignin peroxidase system of white-rot fungus Phanerochaete chrysosporium. FEBS Lett. 1990 Aug 20;269(1):261–263. doi: 10.1016/0014-5793(90)81169-o. [DOI] [PubMed] [Google Scholar]
- Coulter C., Hamilton J. T., Harper D. B. Evidence for the existence of independent chloromethane- and S-adenosylmethionine-utilizing systems for methylation in Phanerochaete chrysosporium. Appl Environ Microbiol. 1993 May;59(5):1461–1466. doi: 10.1128/aem.59.5.1461-1466.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter C., Kennedy J. T., McRoberts W. C., Harper D. B. Purification and Properties of an S-Adenosylmethionine: 2,4-Disubstituted Phenol O-Methyltransferase from Phanerochaete chrysosporium. Appl Environ Microbiol. 1993 Mar;59(3):706–711. doi: 10.1128/aem.59.3.706-711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. L., Raag R., Wariishi H., Gold M. H., Poulos T. L. Crystal structure of lignin peroxidase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):750–754. doi: 10.1073/pnas.90.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Hammel K. E., Jensen K. A., Jr, Mozuch M. D., Landucci L. L., Tien M., Pease E. A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993 Jun 15;268(17):12274–12281. [PubMed] [Google Scholar]
- Hammel K. E., Jensen K. A., Jr, Mozuch M. D., Landucci L. L., Tien M., Pease E. A. Ligninolysis by a purified lignin peroxidase. J Biol Chem. 1993 Jun 15;268(17):12274–12281. [PubMed] [Google Scholar]
- Harper D. B., Buswell J. A., Kennedy J. T., Hamilton J. T. Chloromethane, Methyl Donor in Veratryl Alcohol Biosynthesis in Phanerochaete chrysosporium and Other Lignin-Degrading Fungi. Appl Environ Microbiol. 1990 Nov;56(11):3450–3457. doi: 10.1128/aem.56.11.3450-3457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Piontek K., Glumoff T., Winterhalter K. Low pH crystal structure of glycosylated lignin peroxidase from Phanerochaete chrysosporium at 2.5 A resolution. FEBS Lett. 1993 Jan 4;315(2):119–124. doi: 10.1016/0014-5793(93)81146-q. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüttimann-Johnson C., Salas L., Vicuña R., Kirk T. K. Extracellular Enzyme Production and Synthetic Lignin Mineralization by Ceriporiopsis subvermispora. Appl Environ Microbiol. 1993 Jun;59(6):1792–1797. doi: 10.1128/aem.59.6.1792-1797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]



