Abstract
We tested hypotheses concerning the origin of bird and mammal sex chromosomes by mapping the location of amniote sex-chromosome loci in a salamander amphibian (Ambystoma). We found that ambystomatid orthologs of human X and chicken Z sex chromosomes map to neighboring regions of a common Ambystoma linkage group 2 (ALG2). We show statistically that the proportion of human X and chicken Z orthologs observed on ALG2 is significantly different from the proportion that would be expected by chance. We further show that conserved syntenies between ALG2 and amniote chromosomes are identified as overlapping conserved syntenies when all available chicken (N = 3120) and human (N = 14,922) RefSeq orthologs are reciprocally compared. In particular, the data suggest that chromosomal regions from chicken chromosomes (GGA) Z and 4 and from human chromosomes (HSA) 9, 4, X, 5, and 8 were linked ancestrally. A more distant outgroup comparison with the pufferfish Tetraodon nigroviridis reveals ALG2/GGAZ/HSAX syntenies among three pairs of ancestral chromosome duplicates. Overall, our results suggest that sex chromosomal regions of birds and mammals were recruited from a common ancestral chromosome, and thus our findings conflict with the currently accepted hypothesis of separate autosomal origins. We note that our results were obtained using the most immediate outgroup to the amniote clade (mammals, birds, and other reptiles) while the currently accepted hypothesis is primarily based upon conserved syntenies between in-group taxa (birds and mammals). Our study illustrates the importance of an amphibian outgroup perspective in identifying ancestral amniote gene orders and in reconstructing patterns of vertebrate sex-chromosome evolution.
A classic problem in evolution concerns the origin of sex chromosomes among amniote vertebrates (Ohno 1967). In mammals, females have two identical (XX) sex chromosomes while males have an X and Y (XY). In contrast, birds have a ZZ–ZW determination system wherein females are the heterogametic sex (ZW). The mammalian Y and chicken W chromosomes are conspicuously smaller than their X and Z counterparts and they contain fewer loci. Presumably, these sex-chromosome homologs have undergone extreme, divergent evolution since their recruitment as sex-determining factors, a pattern observed broadly among animals and plants (Ohno 1967; Bull 1983; Lahn et al. 2001; Ayling and Griffin 2002; Charlesworth and Charlesworth 2005; Charlesworth et al. 2005; Khil and Camerini-Otero 2005). Ohno (1967) proposed nearly four decades ago that bird and mammalian sex chromosomes are homologous. Recent comparative genomic analyses have observed that HSAX contains many orthologs of GGA4 genes (Ross et al. 2005) and that GGAZ contains many orthologs of HSA9 genes and fewer orthologs of HSA5 and 8 (Fridolfsson et al. 1998; Burt et al. 1999; Nanda et al. 2002). Because HSAX and GGAZ share few if any orthologs, these comparative data have been interpreted as strong evidence that the sex chromosomes of birds and mammals evolved independently through separate recruitments of bird and mammalian sex chromosomes from independent ancestral autosomes (e.g., Fridolfsson et al. 1998; Nanda et al. 1999, 2000, 2002; Ellegren 2000; Graves et al. 2002; Handley et al. 2004; Kohn et al. 2004, 2006; Khil and Camerini-Otero 2005).
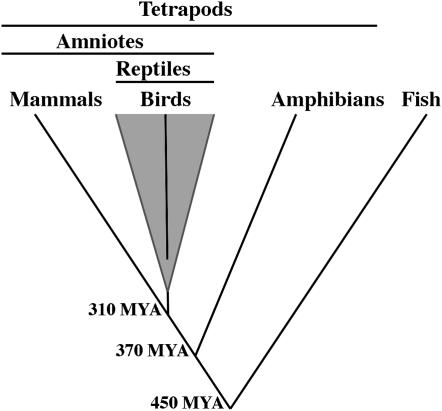
Comparisons between chickens and humans are powerful for identifying features of the ancestral amniote genome that have been conserved in both lineages, but they provide no evolutionary insight about features that have changed within amniote lineages. To determine whether the precursors of GGAZ and HSAX were or were not linked ancestrally, it is necessary to consider the condition of these ancestral regions within an appropriate outgroup species (Stevens 1980; Watrous and Wheeler 1981; Maddison et al. 1984; Futuyma 1998; Martin 2001; Bourque et al. 2005). In general, the most appropriate outgroup is the taxon that is most closely related to the last common ancestor of the clade but not included within the clade (the most proximate outgroup). In the case of amniote/amniote comparisons, amphibians represent the most proximate living outgroup (Figure 1). Until recently, there were few amphibian gene order data available for comparative analyses of vertebrate genome structure (Voss et al. 2001; Smith and Sinclair 2004; Ohta et al. 2006). However, the recently developed genetic linkage map for the salamander genus Ambystoma provides a new outgroup perspective for reconstructing amniote genome evolution (Smith et al. 2005; Smith and Voss 2006). The ambystomatid genome contains relatively few large chromosomes that show extensive synteny conservation with chromosomes from fish and amniote genomes (Smith et al. 2005; Smith and Voss 2006).
Figure 1.—
An abridged phylogeny of the major groups of bony vertebrates. Divergence times were obtained from the literature (Kumar and Hedges 1998; Ruta et al. 2003) Birds represent an ancient reptile lineage that diverged from other reptilian groups ∼220 MYA (Kumar and Hedges 1998).
A few studies of amniote sex-chromosome evolution have used teleost (ray finned) fish to provide an outgroup perspective (Kohn et al. 2004, 2006). The results of these studies have been interpreted as supporting the hypothesis of separate autosomal recruitments because amniote sex-chromosome orthologs are observed to be distributed among several fish chromosomes. However, these studies have not explicitly tested for the presence or absence of the ancestral association of amniote sex chromosomes. Indeed, analyses across deep phylogenetic distances have rarely used statistical approaches to investigate the possibility of conserved syntenies (but see Danchin and Pontarotti 2004). Moreover, it is generally accepted that the ancestor of most teleosts experienced a whole-genome duplication, which was followed by massive losses of paralogous duplicates (Amores et al. 1998; Postlethwait et al. 1998; Jaillon et al. 2004; Woods et al. 2005). Such events, especially in combination with several hundred million years of independent evolution, would be expected to distribute ancestral syntenies among chromosomes.
Interestingly, a recent study of the sex-determining chromosomes of a monotreme (egg laying) mammal seemingly lends support to the idea that Z and W chromosome loci may have been linked in the ancestral amniote genome. The deepest split within the mammalian lineage is between monotremes (platypus and echidna) and therians (all other mammals, i.e., marsupial and placental mammals) (Van Rheede et al. 2006). The platypus X1 chromosome contains many genes from the mammalian X conserved region (XCR) (Graves 1995; Ross et al. 2005) and is linked, via a meiotic translocation chain of five X and five Y chromosomes, to a chromosome that harbors the DMRT1 gene (Grützner et al. 2004; Rens et al. 2004). The gene DMRT1 is located within the sex-determining region of the avian Z chromosome and is a primary candidate for the avian sex-determining gene, along with two W-linked genes: ASW and FET1 (Smith and Sinclair 2004). Currently, it is unclear whether the localization of Z and X orthologs to the platypus sex-determining chromosomes is representative of the condition in the ancestral amniote genome or of rearrangements that were derived after the monotreme/therian divergence (Grützner et al. 2004; Rens et al. 2004; Charlesworth and Charlesworth 2005; Ezaz et al. 2006).
Here, we use the Ambystoma genetic map to provide an outgroup perspective on the origin of bird and mammalian sex chromosomes. We observe that genes from the XCR and GGAZ map to adjacent regions of ALG2, and we further demonstrate that the proportion of sex chromosome orthologies observed on ALG2 is dramatically different from the proportion that would be expected by chance. Further comparisons between chicken and human genomes, and with the draft genome of the pufferfish Tetraodon nigroviridis, support the Ambystoma outgroup perspective and reveal further traces of this common ancestry.
MATERIALS AND METHODS
Linkage mapping and QTL analysis:
Linkage analyses were performed using the previously described mapping panels AxTg (Voss 1995) and WILD2 (Voss and Smith 2005). Primers and probes for all genetic markers have been reported previously (Smith et al. 2005), except for 13 markers on ALG2. Primer sequences, diagnostic polymorphisms, and polymorphism detection assays for these 13 markers are summarized in supplemental Table 1 at http://www.genetics.org/supplemental/. Linkage mapping and association analyses were performed using MapManagerQTXb21 (Meer et al. 2004).
Identification of orthologs:
We identified presumptive orthologies by aligning salamander, human RefSeq, chicken RefSeq, and T. nigroviridis transcripts to human, chicken, and T. nigroviridis genome assemblies. Similarity searches and sequence alignments were accomplished using the program BLAT (Kent 2002). Source sequences for human (International Human Genome Sequencing Consortium 2001), chicken (International Chicken Genome Sequencing Consortium 2004), and T. nigroviridis (Jaillon et al. 2004) (hg17 build 35, galGal3, and tetNig1) genomes were downloaded from the UCSC Genome Browser Gateway (http://genome.ucsc.edu/cgi-bin/hgGateway). Cumulative bitscores were calculated for alignments between transcripts and full genome sequences by summing across presumptive exons. This was accomplished by summing bitscores for otherwise continuous alignments that were interrupted by gaps of 10,000 or fewer bases using the program MapToGenome (Putta et al. 2007). Positions of transcripts within conspecific genomes required an alignment with ≥98% nucleotide sequence identity. A genomic region was considered to be orthologous to a transcript if translated sequences yielded an alignment bitscore of ≥99% of the highest alignment bitscore for that transcript. Positions of orthologous loci were plotted using MapChart 2.1 (Voorrips 2002).
Distribution of orthologies:
Adjusted G-statistics (Sokal and Rohlf 1995) were used to test for nonrandom distribution of sex-chromosome orthologs and orthologs from human and chicken chromosomes that share ancestry with sex chromosomes from the other species (reciprocal amniote sex-chromosome orthologs) on ALG2. We first asked the following question: Are the frequencies of X or Z orthologies on ALG2 significantly higher than expected by chance? The ALG2 loci (the ones that have orthologs) fall into two classes: sex-chromosome orthologs and non-sex-chromosome orthologs (supplemental Table 2 at http://www.genetics.org/supplemental/). The observed numbers of orthologs on ALG2 from the human alignment were 5 from the XCR and 38 from other chromosomes. If ALG2 orthologs were randomly drawn from the human genome, we would expect a proportion of XCR orthologs on ALG2 that approximates the proportion within the human genome. This expected proportion (0.030) was based on the number of genes that are located between 60 and 155 Mb of the X chromosome (N = 810) relative to the total number of genes for the Human Genome Assembly build 36.2 (http://www.ncbi.nih.gov/mapview/stats/BuildStats.cgi?taxid=9606&build=36&ver=2) (N = 28,617), excluding mitochondrial and HSAY genes. A similar statistic was also calculated on the basis of the expected proportion (0.053) of GGAZ genes (N = 840) relative to the total number of genes for the Chicken Genome Assembly build 2.1 (http://www.ncbi.nih.gov/mapview/stats/BuildStats.cgi?taxid=9031&build=2&ver=1) (N = 15,928), excluding mitochondrial and GGAW genes.
On the basis of extensive comparisons between chicken and human genomes (Fridolfsson et al. 1998; Nanda et al. 1999, 2000, 2002; Ellegren 2000; Graves et al. 2002; Handley et al. 2004; Kohn et al. 2004, 2006; Khil and Camerini-Otero 2005), we also asked the question: Is the frequency of reciprocal amniote sex-chromosome orthologies on ALG2 significantly higher than expected by chance? Here, ALG2 loci fall into two classes: reciprocal sex-chromosome orthologs and non-sex-chromosome orthologs. The observed numbers based on the human set (HSAX, 9, 5, 8) are 26 ALG2/sex-chromosome orthologs and 17 ALG2/non-sex-chromosome orthologs. If ALG2 orthologs were randomly drawn from the human genome, we would expect a proportion of HSAX, 9, 5, and 8 orthologs on ALG2 that approximates the proportion within the human genome. This expected proportion (0.165) was based on the number of genes on HSA X, 5, 8, and 9 (N = 4736) relative to the total number of genes for the Human Genome Assembly build 36.1 (N = 28,617). Again, a similar statistic was also calculated on the basis of the expected proportion (0.128) of GGAZ/4 genes (N = 2043) relative to the total number of genes for the Chicken Genome Assembly build 2.1 (N = 15,928).
Adjusted G-statistics (Sokal and Rohlf 1995) were also used to test for nonrandom distribution of T. nigroviridis orthologs among human and chicken chromosomes (supplemental Tables 3–6 at http://www.genetics.org/supplemental/). The frequency of orthologies that were identified on all amniote/T. nigroviridis chromosome pairs was tested for goodness of fit to the frequencies of all orthologs on each of the two chromosomes, relative to the grand total of orthologies that were identified. A similar adjusted G-statistic was also calculated to test for nonrandom distribution of orthologs from the human RefSeq/chicken genome comparison (925 reciprocal amniote sex-chromosome orthologies and 11,116 non-sex-chromosome orthologs; supplemental Table 7) on three ancestrally duplicated pairs of T. nigroviridis chromosomes (H, A, B; sensu Jaillon et al. 2004), relative to all other T. nigroviridis chromosomes.
In practice, some of the statistical analyses that are outlined above can be implemented as a G-test or a permutation test (e.g., Fisher's exact test). Both tests are appropriate for the question at hand and will generally give similar results (Sokal and Rohlf 1995). We have chosen to use G-tests for the analysis of ALG2 orthologies because they permit us to incorporate external data on gene frequencies from the human and chicken genome databases to better approximate sampling probabilities. For consistency, we report G-statistics throughout this article. We note, however, that comparing G-statistics or Fisher's exact tests among different contrasts does not provide insight into the relative strength of association among contrasts. That is, the statistics provide an estimation of the probability of obtaining a given distribution of orthologies under a random sampling scheme, but the values of these statistics are not directly interpretable in a probabilistic sense (Fisher 1938; Goodman and Kruskal 1954; Kendall and Stuart 1967).
RESULTS AND DISCUSSION
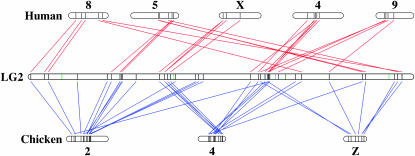
We were able to meiotically map 20 amniote sex-chromosome orthologs to Ambystoma linkage groups. We found that the majority of Z orthologs (85%) mapped to a single Ambystoma linkage group (ALG2) (Figure 2; supplemental Table 2 at http://www.genetics.org/supplemental/). The frequency of Z orthologs on ALG2 was greater than would be expected by chance (Gajd = 6.2, P = 0.013). Many orthologs from the XCR (42%) mapped to neighboring, but nonoverlapping, regions of ALG2 and no more than two X chromosome orthologs were identified on any other Ambystoma linkage group. The frequency of XCR orthologs on ALG2 was also greater than would be expected by chance (Gajd = 6.3, P = 0.009). When we searched all ALG2 genes against the full genome assemblies for human and chicken, we found them to be distributed nonrandomly among human chromosomes (Gajd = 42.6, P = 6.9e−11) and chicken chromosomes (Gajd = 32.9, P = 9.7e−9) that harbor reciprocal amniote sex-chromosome loci. Although some chromosomal associations might be expected by chance, given that the salamander genome is composed of relatively few large chromosomes, statistical analysis shows that the distribution of sex-chromosome orthologies on ALG2 is not likely to have occurred by chance. This pattern of orthologies on ALG2 is consistent with the idea that the X–Y and Z–W chromosomal regions were linked on an ancestral chromosome prior to the divergence of the amphibian and amniote lineages. The pattern of orthologies on ALG2 also provides support for all other conserved syntenies that have been previously identified between amniote sex chromosomes and autosomes (Fridolfsson et al. 1998; Burt et al. 1999; Nanda et al. 2002; International Chicken Genome Sequencing Consortium 2004; Bourque et al. 2005; Ross et al. 2005). The ALG2 region defined by Z orthologs also includes orthologs from HSA 4, 5, 8, and 9 and the ALG2 region defined by the XCR included loci from GGA4. Our comparisons show that many of the gene orders conserved between the sex chromosomes and autosomes of chickens and humans are interspersed but conserved on the same salamander chromosome. Thus, ALG2 apparently retains some of the gene content of an ancestral chromosome that gave rise to the X and Z sex chromosomes.
Figure 2.—
A comparative map of Ambystoma LG2 and syntenic chromosomes from humans and chickens. Vertical bars within ALG2 represent the position of Ambystoma transcripts that yielded an alignment in either the human or the chicken genome. ALG2 loci that do not correspond to orthologs on GGA Z and 4 or on HSA 4, 5, 8, 9, and X are highlighted in green. Red lines connect human/Ambystoma orthologies and blue lines connect chicken/Ambystoma orthologies. The sex chromosomes of human (X) and chicken (Z) are both syntenic with Ambystoma LG2.
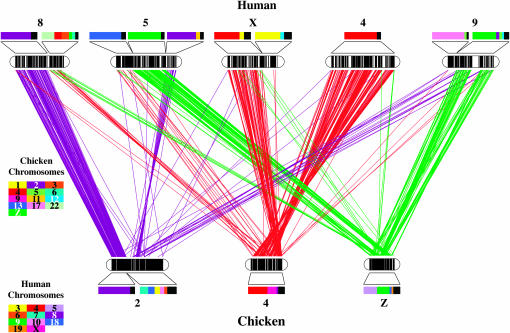
To further test the idea that the sex chromosomes of birds and mammals were derived from the same ancestral chromosome, we identified the location of (N = 14,922) human RefSeq orthologs in the chicken genome and (N = 3,120) chicken RefSeq orthologs in the human genome (supplemental Tables 7 and 8 at http://www.genetics.org/supplemental/). As has been reported previously (Fridolfsson 1998; Burt et al. 1999; Nanda et al. 2002), we identified a large region of conserved synteny between GGAZ and HSA9 (N = 211 loci) (Figure 3) and smaller, but very confined, regions of conserved synteny between GGAZ and HSA8 (N = 19 loci) and between GGAZ and HSA5 (N = 227 loci). In the reciprocal comparison of humans to chickens, we identified a large region of synteny between XCR and GGA4 (N = 272). Thus, when only amniote sex-chromosome loci are considered, there is seemingly no support for the idea of a common autosomal origin for mammalian and bird sex chromosomes, because conserved syntenies are not observed between X and Z loci. However, regions of synteny are observed between GGA4, HSAX, and Y and between GGA4 and human autosomes (HSA 5 and 8) that show synteny with GGA Z (Figure 3). Thus, syntenies that are observed in comparisons of ALG2 and amniote genomes are also observed in comparisons between human and chicken genomes. This pattern supports the idea that amniote chromosomal regions from GGA Z and 4 and from HSA 9, 4, X, 5, and 8 were linked ancestrally.
Figure 3.—
A comparative map of the human and chicken chromosomes that are syntenic with Ambystoma LG2. Vertical bars within chromosomes represent the position of mapped human RefSeq transcripts or chicken orthologs. Lines connect the positions of human/chicken orthologies. Bars above human chromosomes show the proportions of chicken chromosome orthologs that were identified within a given segment, and bars below chicken chromosomes show the proportions of human chromosome orthologs that were identified within a given segment. Note that the chicken chromosomes Z and 4 map to adjacent and overlapping regions of HSA8 and HSA5.
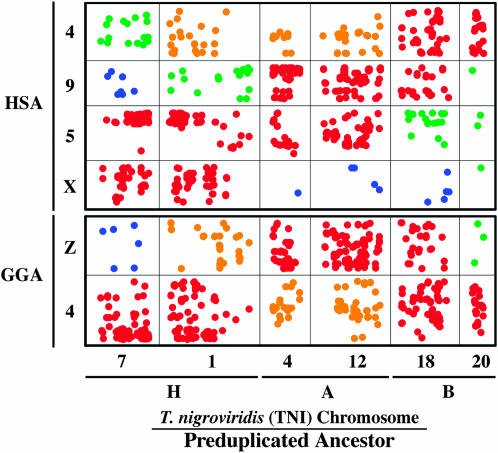
To investigate a deeper outgroup perspective on the evolution of amniote sex chromosomes, we asked the question: Do comparisons with T. nigroviridis provide evidence for deep conservation of HSAX/GGAZ/ALG2 syntenies? Although these comparisons span an additional 670 MY of independent evolution (Kumar and Hedges 1998) and a teleost whole-genome duplication (Amores et al. 1998; Postlethwait et al. 1998; Jaillon et al. 2004; Woods et al. 2005), we detected statistically significant, nonrandom distributions of reciprocal amniote sex-chromosome orthologies among three pairs of T. nigroviridis chromosomes (Figure 4; supplemental Tables 2 and 3 at http://www.genetics.org/supplemental/). These chromosome pairs correspond to the proposed duplicates of ancestral teleost chromosomes A, B, and H (Jaillon et al. 2004). To more directly address the common ancestry of sex-chromosome orthologs among these three duplicate chromosome pairs, we took into consideration the distribution of sex-chromosome orthologs that were identified in comparisons between human RefSeq and the chicken genome (supplemental Table 7). These sex-chromosome orthologs correspond to 1194 human RefSeq genes that fall on HSA 5, 8, 9, or X and correspond to orthologs on GGA 4 or Z. Of these genes, 925 identify orthologs in the T. nigroviridis genome and the overwhelming majority of these (n = 724) fall on the three chromosome pairs (H, A, B) that contain a significant excess of reciprocal amniote sex-chromosome loci (Figure 4). Of 11,116 non-sex-chromosome/T. nigroviridis orthologies, 2906 fall on H, A, and B chromosome pairs and 8210 fall on other T. nigroviridis chromosomes. The distribution of sex-chromosome vs. non-sex-chromosome orthologs on T. nigroviridis H, A, B, chromosome pairs is very unlikely to have occurred by chance (Gajd = 959.8, P = 9.8e−211). Thus, a large fraction the orthologous loci that are associated in the T. nigroviridis duplicates of A, B, and H are from the same amniote chromosomal regions that support ancestral XCR/GGAZ linkage in the salamander genome. Of particular importance is the observation that GGAZ and GGA4 orthologs are linked in both T. nigroviridis and Ambystoma genomes (Figures 2 and 4). Because conserved syntenies also reveal common ancestry of GGA4 and XCR chromosomal regions, the most parsimonious interpretation of T. nigroviridis comparative mapping data is ancestral linkage of Z and X chromosomal regions in the tetrapod and amniote lineages. The distribution of ancestral amniote sex-chromosome regions among different T. nigroviridis chromosomes also reveals the confounding effects of genome duplications and rearrangements that have occurred within the lineage that gave rise to T. nigroviridis subsequent to the diversification of the bony vertebrate (euteleost) ancestor.
Figure 4.—
Oxford plot of the position of amniote sex-chromosome loci in the T. nigroviridis genome. The y-axis represents the relative position of orthologs on human (HSA) and chicken (GGA) chromosomes. The x-axis represents the relative position of orthologs on T. nigroviridis chromosomes. “H”, “A,” and “B” correspond to preduplicated ancestral chromosomes (Jaillon et al. 2004). Cells containing an excess of orthologies are highlighted in red (P < 0.005) and yellow. Cells containing a deficiency of orthologies are highlighted in blue (P < 0.005) and green.
Our comparative analyses provide the first amphibian outgroup perspective on the evolution of amniote sex chromosomes and are most parsimoniously interpreted as evidence for ancestral linkage of XCR and GGAZ regions. However, we recognize that the most parsimonious evolutionary scenario may not always be correct. It is possible (although we think less probable) that these associations are derived from rearrangements that occurred independently within the amniote, amphibian, and fish lineages. Resolution of this issue will necessitate additional comparative data from amphibian outgroups and, to some extent, perspective from the preduplicated fish genome. Ongoing progress toward improving linkage (Smith et al. 2005) and physical (http://genome.jgi-psf.org/Xentr4/Xentr4.home.html) maps for representative amphibian species will likely result in more accurate reconstructions of the ancient events that have structured vertebrate genomes. We note, however, that even with the modest number of Ambystoma/amniote orthologies that are currently mapped, statistical analyses strongly support conserved synteny of ALG2/amniote sex-chromosome orthologs. We therefore expect that additional mapping studies will tend to support our primary findings.
Our study does not resolve the question of what type of sex-determining system was present in the ancestral amniote lineage and how modifications on this ancestral system gave rise to the mammalian XY and avian ZW sex-determining systems. From a theoretical standpoint; it is thought that chromosomal sex-determining systems should evolve from ancestors with environmental (e.g., temperature dependent) sex determination (Bull 1983; Janzen and Krenz 2004; Charlesworth et al. 2005). However, the distribution of ZW and XY systems within fish and amphibian phylogenies suggests that direct transitions between ZW and XY systems may be a relatively common occurrence (reviewed by Ezaz et al. 2006). The unusual sex chromosomes of platypus have been interpreted as evidence supporting the idea that the mammalian XY system evolved from something very similar to the avian ZW (Ezaz et al. 2006; Waters et al. 2007). Here again, information from appropriately positioned outgroups (amphibians) and a whole-genome perspective will be critical for testing alternate hypotheses that platypus sex chromosomes represent an ancestral state vs. a state that was derived independently within the monotreme lineage (Grützner et al. 2004; Rens et al. 2004; Charlesworth and Charlesworth 2005; Ezaz et al. 2006).
In conclusion, our gene mapping data show that amphibian orthologs for loci on chicken and human sex chromosomes are linked in the Ambystoma genome. We interpret this pattern of linkage, which is revealed by including an amphibian outgroup perspective, as a signature of shared ancestry between avian and mammalian sex chromosomes. We believe this signature is retained as a vestige for two reasons:
In comparison to amniotes with chromosomal sex determination, our mapping results show that sex in Ambystoma is determined by a single locus on a chromosome with autosomal characteristics (J. J. Smith and S. R. Voss, unpublished data). Gene orders on ALG2 have not been disrupted by amniote specific rearrangements or mechanisms that are associated with the divergence of dimorphic sex chromosomes (Ohno 1967; Bull 1983; Lahn et al. 2001; Ayling and Griffin 2002; Charlesworth and Charlesworth 2005; Charlesworth et al. 2005; Khil and Camerini-Otero 2005).
In comparison to amniote genome evolution, Ambystoma has experienced relatively lower rates of genome rearrangement and fission (Smith and Voss 2006). The signature of shared sex-chromosome ancestry is difficult to see when comparing only bird and mammalian genomes because mutational processes have fractured and rearranged gene orders within these groups, especially in the mammalian lineage (Burt et al. 1999; Bourque et al. 2005; Smith and Voss 2006).
Our study shows that clarity in comparative vertebrate genomics can be greatly increased by including relevant and phylogenetically well-positioned outgroups like Ambystoma.
Acknowledgments
Comments of several anonymous reviewers substantially improved the quality and clarity of this manuscript. This project was supported by the Kentucky Spinal Cord Injury Research Trust and grant number 5-R24-RR016344-06 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The project was also supported by a National Science Foundation (NSF) CAREER Award (IBN-0242833; IBN-0080112). This project also utilized resources and facilities provided by the Kentucky Bioinformatics Research Infrastructure Network, the Spinal Cord and Brain Injury Research Center, and the NSF-supported Ambystoma Genetic Stock Center (DBI-0443496).
References
- Amores, A., A. Force, Y. L. Yan, L. Joly, C. Amemiya et al., 1998. Zebrafish hox clusters and vertebrate genome evolution. Science 282: 1711–1714. [DOI] [PubMed] [Google Scholar]
- Ayling, L. J., and D. K. Griffin, 2002. The evolution of sex chromosomes. Cytogenet. Genome Res. 99: 125–140. [DOI] [PubMed] [Google Scholar]
- Bourque, G., E. M. Zdobnov, P. Bork, P. A. Pevzner and G. Tesler, 2005. Comparative architectures of mammalian and chicken genomes reveal highly variable rates of genomic rearrangements across different lineages. Genome Res. 15: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull, J. J., 1983. Evolution of Sex Determining Mechanisms. Benjamin Cummings, Menlo Park, CA.
- Burt, D. W., C. Bruley, I. C. Dunn, C. T. Jones, A. Ramage et al., 1999. The dynamics of chromosome evolution in birds and mammals. Nature 402: 411–413. [DOI] [PubMed] [Google Scholar]
- Charlesworth, D., and B. Charlesworth, 2005. Sex chromosomes: evolution of the weird and wonderful. Curr. Biol. 15: R129–R131. [DOI] [PubMed] [Google Scholar]
- Charlesworth, D., B. Charlesworth and G. Marais, 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95: 118–128. [DOI] [PubMed] [Google Scholar]
- Danchin, E. G., and P. Pontarotti, 2004. Statistical evidence for a more than 800-million-year-old evolutionarily conserved genomic region in our genome. J. Mol. Evol. 59: 587–597. [DOI] [PubMed] [Google Scholar]
- Ellegren, H., 2000. Evolution of the avian sex chromosomes and their role in sex determination. Trends Ecol. Evol. 15: 188–192. [DOI] [PubMed] [Google Scholar]
- Ezaz, T., R. Stiglec, F. Veyrunes and J. A. M. Graves, 2006. Relationships between vertebrate ZW and XY sex chromosome systems. Curr. Biol. 16: R736–R743. [DOI] [PubMed] [Google Scholar]
- Fisher, R. A., 1938. Statistical Methods for Research Workers. Oliver & Boyd, Edinburgh.
- Fridolfsson, A. K., H. Cheng, N. G. Copeland, N. A. Jenkins, H.-C. Liu et al., 1998. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Natl. Acad. Sci. USA 95: 8147–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futuyma, D. J., 1998. Evolutionary Biology, Sinauer Associates, Sunderland, MA.
- Goodman, L. A., and W. H. Kruskal, 1954. Measures of association for cross classifications. J. Am. Stat. Assoc. 49: 732–764. [Google Scholar]
- Graves, J. A. M., 1995. The origin and function of the mammalian Y-chromosome and Y-borne genes: an evolving understanding. BioEssays 17: 311–320. [DOI] [PubMed] [Google Scholar]
- Graves, J. A. M., J. Gecz and H. Hameister, 2002. Evolution of the human X—a smart and sexy chromosome that controls speciation and development. Cytogenet. Genome Res. 99: 141–145. [DOI] [PubMed] [Google Scholar]
- Grützner, F., W. Rens, E. Tsend-Ayush, N. El-Mogharbel, P. C. O'Brien et al., 2004. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432: 913–917. [DOI] [PubMed] [Google Scholar]
- Handley, L. L.-J., H. Ceplitis and H. Ellegren, 2004. Evolutionary strata on the chicken Z chromosome: implications for sex chromosome evolution. Genetics 167: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Chicken Genome Sequencing Consortium, 2004. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432: 695–716. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium, 2001. Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- Jaillon, O., J. M. Aury, F. Brunet, J. L. Petit, N. Stange-Thomann et al., 2004. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431: 946–955. [DOI] [PubMed] [Google Scholar]
- Janzen, F. J., and J. G. Krenz, 2004. Phylogenetics: Which was first, TSD or GSD?, pp. 121–130 in Temperature Dependent Sex Determinaiton in Vertebrates, edited by N. V. Lance and A. V. Lance. Smithsonian Books, Washington, DC.
- Kendall, M. G., and A. Stuart, 1967. The Advances Theory of Statistics: Inference and Relationship, Vol. 2. Hafner Publishing, New York.
- Kent, W. J., 2002. BLAT: the BLAST-like alignment tool. Genome Res. 12: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khil, P. P., and R. D. Camerini-Otero, 2005. Molecular features and functional constraints in the evolution of the mammalian X chromosome. Crit. Rev. Biochem. Mol. Biol. 40: 313–330. [DOI] [PubMed] [Google Scholar]
- Kohn, M., H. Kehrer-Sawatzki, W. Vogel, J. A. M. Graves and H. Hameister, 2004. Wide genome comparisons reveal the origins of the human X chromosome. Trends Genet. 20: 598–603. [DOI] [PubMed] [Google Scholar]
- Kohn, M., J. Hogel, W. Vogel, P. Minich, H. Kehrer-Sawatzki et al., 2006. Reconstruction of a 450-My-old ancestral vertebrate protokaryotype. Trends Genet. 22: 203–210. [DOI] [PubMed] [Google Scholar]
- Kumar, S., and B. Hedges, 1998. A molecular timescale for vertebrate evolution. Nature 392: 917–920. [DOI] [PubMed] [Google Scholar]
- Lahn, B. T., N. M. Pearson and K. Jegalian, 2001. The human Y chromosome, in the light of evolution. Nat. Rev. Genet. 2: 207–216. [DOI] [PubMed] [Google Scholar]
- Maddison, W. P., M. J. Donoghue and D. R. Maddison, 1984. Outgroup analysis and parsimony. Syst. Zool. 33: 83–103. [Google Scholar]
- Martin, A., 2001. The phylogenetic placement of Chondrichthyes: inferences from analysis of multiple genes and implications for comparative studies. Genetica 111: 349–357. [DOI] [PubMed] [Google Scholar]
- Meer, J. M., R. H. Cudmore, Jr. and K. F. Manly, 2004. MapManager QTX. http://ww.mapmanager.org/mmQTX.html.
- Nanda, I., Z. Shan, M. Schartl, D. W. Burt, M. Koehler et al., 1999. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 21: 258–259. [DOI] [PubMed] [Google Scholar]
- Nanda, I., E. Zend-Ajusch, Z. Shan, F. Grutzner, M. Schartl et al., 2000. Conserved synteny between the chicken Z and human 9 chromosomes includes the male regulatory gene DMRT1: a comparative (re)view on avian sex-determination. Cytogenet. Cell Genet. 89: 67–78. [DOI] [PubMed] [Google Scholar]
- Nanda, I., T. Haaf, M. Schartl, M. Schmid and D. W. Burt, 2002. Comparative mapping of Z-orthologous genes in vertebrates: implications for the evolution of avian sex chromosomes. Cytogenet. Genome Res. 99: 178–184. [DOI] [PubMed] [Google Scholar]
- Ohno, S., 1967. Sex chromosomes and sex linked genes, pp. 1–192 in Monographs on Endocrinology, Vol. 1, edited by A. Labhart, T. Mann and L. T. Samuels. Springer-Verlag, New York.
- Ohta, Y., W. Goetz, M. Z. Hossain, M. Nonaka and M. F. Flajnik, 2006. Ancestral organization of the MHC revealed in the amphibian Xenopus. J. Immunol. 176: 3674–3685. [DOI] [PubMed] [Google Scholar]
- Postlethwait, J. H., Y. L. Yan, M. A. Gates, S. Horne, A. Amores et al., 1998. Vertebrate genome evolution and the zebrafish gene map. Nat. Genet. 18: 345–349. [DOI] [PubMed] [Google Scholar]
- Putta, S., J. J. Smith, C. Staben and S. R. Voss, 2007. MapToGenome: a comparative genomic tool that aligns transcript maps to aequenced genomes. Evol. Bioinform. Online 2007: 15–25. [PMC free article] [PubMed]
- Rens, W., F. Grützner, P.C. O'Brien, H. Fairclough, J. A. M. Graves et al., 2004. Resolution and evolution of the duck-billed platypus karyotype with an X1Y1 X2Y2 X3Y3 X4Y4 X5Y5 male sex chromosome constitution. Proc. Natl. Acad. Sci. USA 101: 16257–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, M. T., D. V. Grafham, A. J. Coffey, S. Scherer, K. McLay et al., 2005. The DNA sequence of the human X chromosome. Nature 434: 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta, M., M. I. Coates and D. J. Quicke, 2003. Early tetrapod relationships revisited. Biol. Rev. Camb. Philos. Soc. 78: 251–345. [DOI] [PubMed] [Google Scholar]
- Smith, C. A., and A. H. Sinclair, 2004. Sex determination: insights from the chicken. BioEssays 26: 120–132. [DOI] [PubMed] [Google Scholar]
- Smith, J. J., and S. R. Voss, 2006. Gene order data from a model amphibian (Ambystoma): new perspectives on vertebrate genome structure and evolution. BMC Genomics 7: 219. [DOI] [PMC free article] [PubMed]
- Smith, J. J., D. K. Kump, J. A. Walker, D. M. Parichy and S. R. Voss, 2005. A comprehensive expressed sequence tag linkage map for tiger salamander and Mexican axolotl: enabling gene mapping and comparative genomics in Ambystoma. Genetics 171: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, F. J., and R. R. Rohlf, 1995. Biometry: The Principles and Practice of Statistics in Biological Research, Ed. 3. W. H. Freeman, New York.
- Stevens, P. F., 1980. Evolutionary polarity of character states. Annu. Rev. Ecol. Syst. 11: 333–358. [Google Scholar]
- van Rheede, T., T. Bastiaans, D. N. Boone, S. B. Hedges, W. W. de Jong et al., 2006. The platypus is in its place: nuclear genes and indels confirm the sister group relation of monotremes and therians. Mol. Biol. Evol. 23: 587–597. [DOI] [PubMed] [Google Scholar]
- Voorrips, R. E., 2002. MapChart. http://www.plant.wageningenur.nl/products/mapping/MapChart.
- Voss, S. R., 1995. Genetic basis of paedomorphosis in the axolotl Ambystoma mexicanum: a test of the single gene hypothesis. J. Hered. 86: 441–447. [Google Scholar]
- Voss, S. R., and J. J. Smith, 2005. Evolution of salamander life cycles: a major-effect QTL contributes to discrete and continuous variation for metamorphic timing. Genetics 170: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss, S. R., J. J. Smith, D. M. Gardiner and D. M. Parichy, 2001. Conserved vertebrate chromosome segments in the large salamander genome. Genetics 158: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, P. D., M. C. Wallis and J. A. M. Graves, 2007. Mammalian sex: origin and evolution of the Y chromosome and SRY. Semin. Cell Dev. Biol. (in press). [DOI] [PubMed]
- Watrous, L. E., and Q. E. Wheeler, 1981. The out-group comparison method of character analysis. Syst. Zool. 30: 1–11. [Google Scholar]
- Woods, I. G., C. Wilson, B. Friedlander, P. Chang, D. K. Reyes et al., 2005. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 15: 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]