Abstract
SDF-9 is a modulator of Caenorhabditis elegans insulin/IGF-1 signaling that may interact directly with the DAF-2 receptor. SDF-9 is a tyrosine phosphatase-like protein that, when mutated, enhances many partial loss-of-function mutants in the dauer pathway except for the temperature-sensitive mutant daf-2(m41). We propose that SDF-9 stabilizes the active phosphorylated state of DAF-2 or acts as an adaptor protein to enhance insulin-like signaling.
IN an environment favorable for reproduction, Caenorhabditis elegans develops directly into an adult through four larval stages (L1–L4). Under conditions of overcrowding, limited food, or high temperature, larvae arrest development at the second molt to form dauer larvae (Cassada and Russell 1975). Dauer larvae can remain in diapause for months, essentially not aging, until conditions improve (Klass and Hirsh 1976). The signaling pathways that control the developmental switch have been revealed by studying two broad classes of mutants: those that block dauer formation (Daf-d, dauer formation defective) and those that result in constitutive dauer or dauer-like arrest (Daf-c), even when conditions are favorable (reviewed by Riddle and Albert 1997).
The daf genes encode elements of pathways conserved in humans, including insulin/IGF-1 (IIS), transforming growth factor-β (TGF-β), target of rapamycin, and guanylate cyclase/G-protein-mediated pathways (reviewed by Hafen 2004; Levy and Hill 2006). In addition to the Daf-c phenotype, mutants with reduced IIS signaling also display an adult life-span extension (Age) phenotype (Kenyon et al. 1993; Larsen et al. 1995).
Higher growth temperatures favor dauer formation, as observed in wild-type strains (C. elegans N2 and wild-type C. briggsae) exposed to exogenous dauer pheromone (Golden and Riddle 1984a; Jeong et al. 2005; Butcher et al. 2007) and in hypomorphic Daf-c mutants, which convey increased pheromone sensitivity (Golden and Riddle 1984b). The concentration of dauer pheromone indicates population density. Among the Daf-c mutants, null mutants of TGF-β pathway genes convey a temperature-sensitive (ts) phenotype, whereas severe daf-2 or age-1 mutants (IIS pathway) arrest at the dauer stage nonconditionally (Larsen et al. 1995; Ren et al. 1996). The IIS pathway is essential for larval maturation beyond the dauer stage, whereas the TGF-β pathway is essential only at higher temperatures as long as the IIS pathway is intact. Most Daf-c alleles are temperature sensitive not as a result of a thermolabile protein product but because temperature is an input into dauer formation (Golden and Riddle 1984a). One truly temperature-sensitive allele has been described, daf-2(m41), which is wild type at 15°, but severe at 25° (Gems et al. 1998). This is unlike other daf-2 alleles, which have partially penetrant Daf-c phenotypes at 15° that correlate with their severity at 20°, indicating that they are hypomorphic.
Forward mutagenesis screens have been useful, not only for understanding dauer biology, but also because the cloning of Daf-c and Daf-d genes has identified novel members of these conserved pathways (Georgi et al. 1990; Ren et al. 1996; Ogg et al. 1997; Patterson et al. 1997; Paradis and Ruvkun 1998; Da Graca et al. 2004). There are likely to be more genes involved that modulate or coordinate these pathways (Tewari et al. 2004). There is also value in creating new alleles for genes already known to be involved in the dauer pathways. Transposon insertion mutants allow isolation of knockout mutations or in situ knockin alterations to the gene (Plasterk and Groenen 1992; Barrett et al. 2004). In the mut-2 (mutator) genetic background, mobilized transposons of the Tc family insert into or near genes preferentially at consensus sites (Collins et al. 1987). We used mut-2 in a screen to identify novel Daf-c mutants and to obtain transposon insertion alleles for genes already known (Caldicott 1995).
Isolation and mapping of m708:
One nonconditional Daf-c mutant from the mut-2 screen failed to complement daf-2(e1370). Upon subsequent backcrossing, it was determined to contain not only a ts allele of daf-2, m637, but also an independently segregating daf-2 enhancer, m708. The m708 homozygous single mutant exhibited only a weak egg laying phenotype and no evidence of Daf-c, Hid (Daf-c at 27°), neuronal dye filling, or Age phenotypes previously associated with Daf mutants (Malone and Thomas 1994; Larsen et al. 1995; Ailion and Thomas 2000).
Three-factor mapping placed m708 on chromosome V to the right of unc-51. Polymerase chain reaction (PCR) amplification of six candidate genes using gene-specific primers revealed that one gene, sdf-9 (synthetic dauer formation), had a 1.2-kb transposon insertion in exon 4. On the basis of dauer formation on NGM agar plates without cholesterol (Ohkura et al. 2003), m708 failed to complement sdf-9(ut163). The insert is contained between C515 and T516 of the coding sequence and is nine bases to the left of the lesion in ut163 and nine bases to the right of the lesion in the ut169 and ut174 alleles (Ohkura et al. 2003). The m708 open reading frame ends with an amber stop 24 codons into the insert. No sdf-9 mRNAs were detected in either of two sdf-9(m708) RNA preparations, but we cannot eliminate the possibility that an altered gene product may be produced.
Cemar1 transposition:
Unexpectedly, sdf-9(m708) did not contain a transposon normally mobilized by mut-2 (i.e., Tc1 or Tc5). Instead, it contained Cemar1 (C. elegans Mariner 1), found in 66 copies in the haploid genome of the N2 strain, but not previously reported to be mobile (Witherspoon and Robertson 2003). Cemar1 was originally identified by the repeat identifying program RECON (Bao and Eddy 2002) and is currently listed by WormBase as Ce000178 (release WS172; http://www.wormbase.org/).
The sequence of the inserted element differed from the consensus by an 11-bp deletion and a T > C mutation. The only copy of Cemar1 with the corresponding sequence is located on chromosome V, 10 Mbp to the left of sdf-9 in an intron of the gene D1054.5, indicating that the transposon inserted in sdf-9(m708) originated from this copy. The parental mut-2 strain used for the mutant screen contained no transposon in sdf-9, whereas the original isolate of sdf-9(m708) still contained the parent transposon in D1054.5 as well as the insert in sdf-9(m708). Most copies of Cemar1 encode a functional transposase (Witherspoon and Robertson 2003), the expression of which has been observed in at least two expression studies (Kim et al. 2001; Murphy et al. 2003). We conclude that Cemar1 was mobilized in the mut-2 background, and other uncharacterized mutants isolated in this background may bear insertions of Cemar1 rather than members of the Tc family.
Epistatic analysis of sdf-9 and daf-c mutants:
Since sdf-9(m708) enhanced daf-2(m637), double mutants were created with other representative alleles of daf-2 to determine if the enhancement is allele specific. As was the case with daf-2(m637), the daf-2(e1370); sdf-9(m708) double mutant showed a much stronger Daf-c phenotype compared to daf-2(e1370) (Table 1). Double mutants between sdf-9(m708) and the strong ts daf-2 alleles, e979 (Table 1) and m637 (data not shown), were nonconditional, forming only nonrecovering dauer larvae at 15°.
TABLE 1.
Interaction between sdf-9 and daf-2
| 15°
|
20°
|
22°
|
23°
|
25°
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Daf-c | N | Daf-c | N | Daf-c | N | Daf-c | N | Daf-c | N |
| daf-2(m41) | 0 | 129 | 10 ± 5 | 365 | 33 ± 3 | 393 | 100 | >500a | 100 | 131 |
| sdf-9(m708) | 0 | 300 | 0 | 370 | 0 | 280 | — | — | 0 | 140 |
| m41; m708 | 0 | 72 | 5 ± 6 | 133 | 30 ± 5 | 332 | 100 | >500a | 100 | 96 |
| sdf-9(ut163) | 0 | 258 | 0 | 667 | 0 | 321 | — | — | 17 ± 2 | 110 |
| m41; ut163 | 0 | 340 | 4 ± 2 | 339 | 31 ± 6 | 395 | — | — | 100 | 126 |
| daf-2(e1368) | — | — | 1 ± 1 | 195 | 6 ± 1 | 388 | 8 ± 3 | 250 | — | — |
| e1368; m708 | — | — | 60 ± 17 | 424 | 89 ± 6 | 340 | 100 | 220 | — | — |
| daf-2(e1365) | — | — | 1 ± .3 | 381 | 4 ± 2 | 544 | 16 ± 8 | 1277 | — | — |
| e1365; m708 | — | — | 14 ± 4 | 280 | 53 ± 15 | 131 | 100 | 1120 | — | — |
| daf-2(e1370) | 0 | 683 | 3 ± .4 | 1131 | — | — | — | — | — | — |
| e1370; m708 | 92 ± 3 | 628 | 100 | >500a | — | — | — | — | — | — |
| e1370; ut163 | 0 | 766 | 50 ± 4 | 959 | — | — | — | — | — | — |
| daf-2(e979) | 10b | 30 | — | — | — | — | — | — | — | — |
| e979; m708 | 100b | 30 | — | — | — | — | — | — | — | — |
Values are percentage of dauer larvae ± standard error, including dauer and dauer-like larvae counted on the first day of adulthood for non-dauer siblings. Populations were synchronized by hatching alkaline hypochlorite-treated embryos in M9 buffer at room temperature for 24 hr and then transferring them to NGM plates with Escherichia coli OP50. N, population size. Alleles are listed in order of severity except m41, which is listed first because of its unique phenotype. Strains were genotyped as follows: PCR for sdf-9(m708), sequencing for daf-2(m41) and sdf-9(ut163) and by phenotype for all other daf-2 alleles.
Based on visual inspection of multiple samples (>500 animals) with no larvae growing past dauer.
Includes genotyped progeny from one daf-2(e979)/daf-2(e979); sdf-9(m708)/+, including 12 dauers, 35 nonrecovering dauers (allowed to recover at 15° for 3 days after cold shock at 4° overnight) and 53 adults (χ2 P-value <0.001).
We also tested daf-2(e1365) and daf-2(e1368), two alleles with very weak Daf-c phenotypes, and daf-2(m41), a unique ts mutant exhibiting no Daf-c or Age phenotype at 15°, but a stronger Daf-c phenotype at 22.5° than other daf-2 alleles that are Age at 15° (Gems et al. 1998). At 20°, 22°, and 23°, m708 strongly enhanced both e1365 and e1368. However, m708 did not enhance m41 at any temperature at which enhancement might be detected (Table 1).
To investigate possible sdf-9 allele specificity in the interaction with daf-2, we made sdf-9(ut163) double mutants with daf-2(m41) and daf-2(e1370). As was the case with m708, ut163 enhanced daf-2(e1370) dauer formation but not daf-2(m41) (Table 1). The ut163 allele appears to be ts because it has a very weak phenotype at 15° [no enhancement of daf-2(e1370)], a moderate phenotype at 20° (weaker enhancement than sdf-9(m708)), and a stronger Daf-c phenotype than m708 at 25° (Table 1). Ohkura et al. (2003) reported that the ut163 Daf-c phenotype was the second strongest among the tested sdf-9 alleles at 25° but the weakest allele at 20° on NGM plates lacking cholesterol, also indicating that the ut163 protein may be thermolabile.
Since sdf-9 enhanced all of the daf-2 alleles tested except for m41, it could be judged to fall into a parallel dauer formation pathway. sdf-9(m708) also enhanced daf-7(e1372) and daf-8(m85), which encode components of the TGF-β pathway (Table 2). In fact, double mutants between sdf-9(m708) and the type I and type II TGF-β receptors, daf-1(m40) and daf-4(e1364), respectively, constitutively formed dauer larvae that could not recover at 15° (data not shown). Furthermore, the Daf-c phenotype of daf-11, encoding a transmembrane guanylate cyclase (Birnby et al. 2000), was enhanced by sdf-9. daf-11(m47) sdf-9(m708) exhibited strong dauer formation at 15° (Table 2), with many dauer larvae unable to resume development. Taken together, it appears that SDF-9 may work in parallel to the TGF-β and guanylate cyclase pathways.
TABLE 2.
Effect of sdf-9 on mutants in other dauer pathways
| 15°
|
||
|---|---|---|
| Genotype | Daf-ca | N |
| daf-7(e1372) | 4 ± 2 | 465 |
| e1372; m708 | 64 ± 3 | 177 |
| daf-8(m85) | 1 ± 0 | 481 |
| m85; m708 | 63 ± 6 | 175b |
| daf-11(m47) | 4 ± 2 | 330 |
| m47 m708 | 85 ± 6 | 106 |
Percentage of constitutive dauer formation ± standard error, with populations scored as in Table 1. Genotype was confirmed by phenotype for daf-7(e1372), daf-8(m85), and daf-11(m47) and by PCR for sdf-9(m708).
Approximately one-quarter of the animals in this sample were multivulva, small, embryonic lethal, grew slowly, or had other morphological defects. These were not included in the counts.
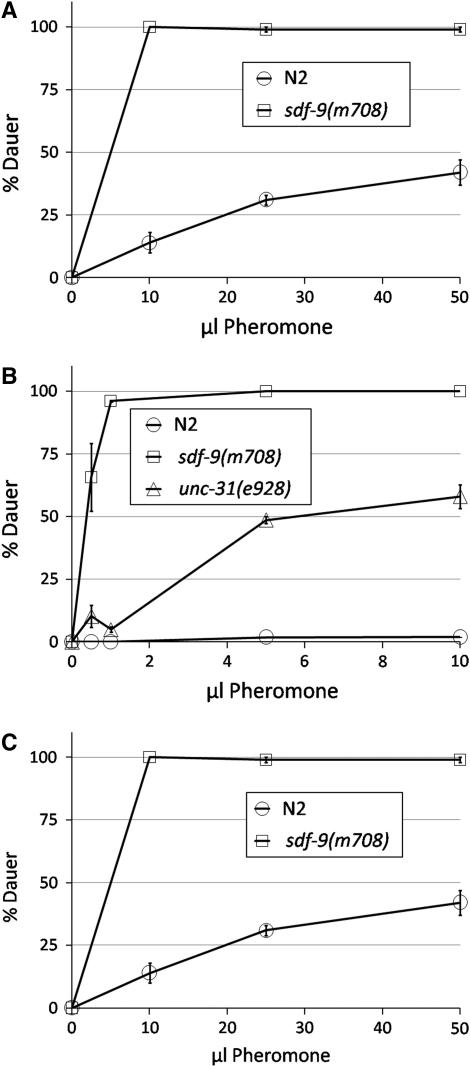
sdf-9 not only enhanced the phenotypes of all tested Daf-c mutants except for daf-2(m41), but also proved to be hypersensitive to dauer pheromone, as are the Daf-c mutants (Golden and Riddle 1984b). sdf-9(m708) formed far more dauer larvae than N2 at all temperatures and concentrations of pheromone tested (Figure 1). We then subjected sdf-9(m708); daf-d double mutants from the IIS (daf-16) and TGF-β (daf-3) pathways to dauer pheromone at 2.5 μl/plate to see which pathway mediates the hypersensitivity. daf-16(m26); sdf-9(m708) failed to form dauer larvae in response to pheromone (N = 114), but sdf-9(m708); daf-3(e1376) formed only slightly fewer dauer larvae (76 ± 6%, N = 119) than sdf-9(m708) alone (100%, N = 124). Neither the daf-3(e1376) nor the daf-16(m26) single mutant formed any dauer larvae (N = 116 and 102, respectively). This suggests that SDF-9 modulates the IIS pathway, since daf-16(+) is required for sdf-9 to manifest its effect, whereas daf-3(+) is not required.
Figure 1.—
Response of sdf-9(m708) to dauer pheromone. The pheromone extract and plates were made as previously described (Golden and Riddle 1984b) and used in the amounts given. (A and B) Each data point ± standard error represents results from two or three plates, each started with ∼40 eggs laid in situ by three gravid adults, which were subsequently removed. Plates were scored for dauer formation on the first day of adulthood. At 25.5°, sdf-9(m708) showed increased sensitivity to pheromone for repeated experiments even when compared to unc-31(e928), previously reported to be sensitive to pheromone at 25.4° (Ailion and Thomas 2000). (C) Using the same method, hypersensitivity to pheromone was also observed at 20°.
daf-16 suppression of sdf-9 hypersensitivity to dauer pheromone is dominant. We crossed daf-16(m26)/+; sdf-9(m708)/+ males to sdf-9(m708); dpy-3(e27) double mutants and exposed the progeny to 2.5 μl of dauer pheromone. If daf-16 were a recessive suppressor, we would expect 50% dauer larvae among the hermaphrodite progeny, but we observed only 27 ± 7% (N = 64) for the line used in the pheromone assay and 21 ± 6% (N = 63) for another isolate of the same genotype. This is compared to 100% dauer larvae in the control sdf-9(m708) strain (N = 120) and 0% for N2 (N = 114). Since a 50% reduction in daf-16(+) gene dosage suppresses dauer formation, high levels of DAF-16 activity must be required for expression of the sdf-9 mutant phenotype.
Enhancement of daf-2 by sdf-9(m708) was also semidominant. As shown in Table 1, 100% of the progeny of daf-2(e1368); sdf-9(m708) formed dauer larvae compared to 8% dauer larvae for daf-2(e1368) at 23°. If sdf-9(m708) were recessive, the expected number of dauer larvae segregated from daf-2(e1368); sdf-9(m708)/+ heterozygotes would be 31% [= 25% sdf-9(−/−) + 8% × 75% sdf-9(+/− and +/+) = 31%], but we observed 41 ± 3% (N = 624). We conclude that sdf-9(m708) is a semidominant enhancer of daf-2 (P-value from χ2 = 4.5 × 10−4). A 50% reduction in sdf-9(+) gene dosage enhances the phenotype of weak daf-2 mutants. Both SDF-9 and DAF-16 are points of fine tuning for insulin-like signaling.
To test whether sdf-9 mutants may be long lived, as are the Daf-c mutants in the IIS pathway (Kenyon et al. 1993; Larsen et al. 1995), we compared the life spans of sdf-9(m708) with N2 and daf-2(e1370); sdf-9(m708) with daf-2(e1370). In agreement with the previous results for sdf-9 (Ohkura et al. 2003; Hu et al. 2006), we saw no increase in the life span of sdf-9(m708) relative to N2. Similarly, sdf-9(m708) had no effect on the life span of daf-2 (data not shown), despite enhancing its Daf-c phenotype. SDF-9 may function to enhance IIS primarily during larval development, and not during adulthood. By contrast, treatment of wild-type adults with daf-2 RNA interference is sufficient to increase longevity (Dillin et al. 2002).
Slow maturation of Daf-c mutants to the adult stage at intermediate temperatures results from entry into an L2d-like state (the L2d is the pre-dauer L2 larva) with a delayed second molt (Swanson and Riddle 1981). ut163 fully suppressed the slow growth of daf-2(m41) at 20°, whereas m708 had no effect (Table 3). At 22°, both alleles partially suppressed the slow-growth phenotype. Suppression of the L2d delay would suggest a gain of SDF-9 function at the intermediate temperatures, but this is not supported by the dauer formation data (Table 1), which show no obvious suppression of the Daf-c phenotype at 20° or 22°. Instead, suppression of the slow-growth phenotype suggests that SDF-9 may interact with other pathways required for growth. For example, sterol deprivation has already been shown to enhance the weak Daf-c phenotype of sdf-9 (Ohkura et al. 2003).
TABLE 3.
Maturation time
| Genotype | 20° | 22° |
|---|---|---|
| N2 | 64 | 56 |
| daf-2(m41) | 104 | 96 |
| sdf-9(m708) | 64 | 56 |
| sdf-9(ut163) | 64 | 56 |
| m41; m708 | 104 | 88 |
| m41; ut163 | 64 | 80 |
Populations of 50–120 animals/plate (8–10 plates/genotype) were synchronized as in Table 1 prior to transfer to NGM agar plates with E. coli at 20° and 22°. They were observed every 8 hr until all 8–10 plates for each sample had eggs present. Within each strain there was no variation observed among plates.
Model for SDF-9 function:
We isolated an allele of sdf-9 as an enhancer of daf-2. This gene was previously detected as an enhancer of akt-1 (Hu et al. 2006) and unc-31 (Ohkura et al. 2003). We found that sdf-9 enhanced all Daf-c mutants tested, except daf-2(m41). We propose that the allele specificity of interaction with daf-2 and the requirement for daf-16(+), not daf-3(+), for SDF-9 function demonstrate genetically that SDF-9 modulates the IIS pathway, at least in larvae. These data support previous interpretations of SDF-9 function (Ohkura et al. 2003; Hu et al. 2006), which were based on its identity as a tyrosine-phosphatase-like protein lacking the necessary catalytic cysteine residue and membrane-bound subcellular localization. These authors suggested that SDF-9 might act along with EAK-6 (another likely inactive tyrosine phosphatase that enhances the akt-1 mutant phenotype) to bind the DAF-2-activating phosphotyrosine, but they did not test for genetic or molecular interaction with daf-2.
Tyrosine kinase receptors like DAF-2 function via ligand binding, dimerization, activation by trans-autophosphorylation, phosphorylation of target proteins, and deactivation by a tyrosine phosphatase (reviewed by Romano 2003). It is possible that SDF-9 enhances IIS signaling by protecting phosphorylated DAF-2 from inactivation by a tyrosine phosphatase, or it may act as an adaptor protein to enhance binding of a DAF-2 target to the DAF-2 kinase. Hypomorphic daf-2 alleles would be sensitive to loss of SDF-9 as long as DAF-2 is able to trans-autophosphorylate.
The daf-2(m41) mutation is a G-to-A substitution that changes a glycine to glutamic acid at position 383 (Yu and Larsen 2001). The glycine is part of a Caenorhabditis conserved glycine–proline turn motif adjacent to a conserved cysteine in the cysteine-rich region in the extracellular domain. This structural change may disrupt a disulfide bond formed by the conserved cysteine and could make the overall structure of the daf-2(m41) gene product unstable or unable to dimerize at higher temperatures. Mutations in a similar domain of the EGFR protein cause an inability to dimerize, preventing trans-autophosphorylation (Macdonald et al. 2006). Lack of trans-autophosphorylation would render SDF-9 unable to modify m41 activity, so loss of SDF-9 function would not affect the m41 phenotype. Alternatively, thermolability of the daf-2(m41) protein may prevent binding of SDF-9 to DAF-2, also resulting in no enhancement of daf-2(m41) by sdf-9(m708). We propose that all the hypomorphic daf-2 alleles that are enhanced by sdf-9(m708) and sdf-9(ut163) trans-autophosphorylate at some level.
Formally, SDF-9 could bind to a phosphorylated target of DAF-2, rather than to DAF-2 itself. However, one would not expect daf-2 allele specificity in that case, since the enhancement would be affected only by the phosphorylation state of the particular DAF-2 target. This explanation seems far less likely than our posited lack of m41 protein dimerization.
STYX family proteins have tyrosine phosphatase domains that are phosphatase inactive (Wishart and Dixon 1998). They have been shown to bind proteins with phosphorylated serine, threonine, or tyrosine to act as adaptor proteins or to protect the phosphorylated tyrosine. Co-incubation of the mammalian STYX protein Sbf1 with SUV39H1, a mammalian ortholog of Drosophila Su(var)3-9 (suppressor of variegation), was shown to stabilize the phosphorylated state of SUV39H1, whereas engineering Sbf1 to restore catalytic phosphatase activity eliminated such stabilization (Firestein et al. 2000). In C. elegans, the STYX protein IDA-1 enhances daf-2(e1370), apparently by functioning in several neurons likely to regulate insulin secretion (Cai et al. 2004).
On the basis of the genetic evidence provided by the daf-2 allele specificity of interactions with sdf-9 and the requirement for daf-16(+) to exhibit the sdf-9 pheromone sensitivity, we propose that SDF-9 functions in the IIS pathway and binds to DAF-2 at one or more of the activating phosphotyrosines to enhance DAF-2 signaling during larval development. Loss of SDF-9 function reduces insulin-like signaling to enhance the phenotype of Daf-c mutants, even those in parallel pathways. If this interaction is conserved in other species, such phosphatase-like proteins could potentially be useful targets of novel therapies for diseases such as type 2 diabetes in humans.
Acknowledgments
We thank Ian Caldicott for initial isolation of the daf-2(m637); sdf-9(m708) strain and Marco Gallo and Nigel O'Neil for helpful discussions. The Caenorhabditis Genetics Center provided the sdf-9(ut163) strain. This work was supported by grants from the National Institutes of Health and the Canadian Institutes for Health Research to D.L.R. V.L.J. was supported by a fellowship from the Natural Sciences and Engineering Research Council of Canada and is a Junior Graduate Trainee of the Michael Smith Foundation for Health Research.
References
- Ailion, M., and J. H. Thomas, 2000. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics 156: 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, Z., and S. R. Eddy, 2002. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 12: 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, P. L., J. T. Fleming and V. Gobel, 2004. Targeted gene alteration in Caenorhabditis elegans by gene conversion. Nat. Genet. 36: 1231–1237. [DOI] [PubMed] [Google Scholar]
- Birnby, D. A., E. M. Link, J. J. Vowels, H. Tian, P. L. Colacurcio et al., 2000. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 155: 85–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher, R. A., M. Fujita, F. C. Schroeder and J. Clardy, 2007. Small molecule signaling of dauer formation in C. elegans. Nat. Chem. Biol. 3: 420–422. [DOI] [PubMed] [Google Scholar]
- Cai, T., T. Fukushige, A. L. Notkins and M. Krause, 2004. Insulinoma-associated protein IA-2, a vesicle transmembrane protein, genetically interacts with UNC-31/CAPS and affects neurosecretion in Caenorhabditis elegans. J. Neurosci. 24: 3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldicott, I. M., 1995. Non-conditional dauer and dauer-like mutants of Caenorhabditis elegans. Ph.D. Thesis, University of Missouri, Columbia, MO.
- Cassada, R. C., and R. L. Russell, 1975. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326–342. [DOI] [PubMed] [Google Scholar]
- Collins, J., B. Saari and P. Anderson, 1987. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature 328: 726–728. [DOI] [PubMed] [Google Scholar]
- Da Graca, L. S., K. K. Zimmerman, M. C. Mitchell, M. Kozhan-Gorodetska, K. Sekiewicz et al., 2004. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF beta pathway to regulate C. elegans dauer development. Development 131: 435–446. [DOI] [PubMed] [Google Scholar]
- Dillin, A., D. K. Crawford and C. Kenyon, 2002. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science 298: 830–834. [DOI] [PubMed] [Google Scholar]
- Firestein, R., X. Cui, P. Huie and M. L. Cleary, 2000. Set domain-dependent regulation of transcriptional silencing and growth control by SUV39H1, a mammalian ortholog of Drosophila Su(var)3–9. Mol. Cell. Biol. 20: 4900–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems, D., A. J. Sutton, M. L. Sundermeyer, P. S. Albert, K. V. King et al., 1998. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi, L. L., P. S. Albert and D. L. Riddle, 1990. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61: 635–645. [DOI] [PubMed] [Google Scholar]
- Golden, J. W., and D. L. Riddle, 1984. a The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102: 368–378. [DOI] [PubMed] [Google Scholar]
- Golden, J. W., and D. L. Riddle, 1984. b A pheromone-induced developmental switch in Caenorhabditis elegans: temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc. Natl. Acad. Sci. USA 81: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen, E., 2004. Cancer, type 2 diabetes, and ageing: news from flies and worms. Swiss Med. Wkly. 134: 711–719. [DOI] [PubMed] [Google Scholar]
- Hu, P. J., J. Xu and G. Ruvkun, 2006. Two membrane-associated tyrosine phosphatase homologs potentiate C. elegans AKT-1/PKB signaling. PLoS Genet. 2: e99. [DOI] [PMC free article] [PubMed]
- Jeong, P. Y., M. Jung, Y. H. Yim, H. Kim, M. Park et al., 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433: 541–545. [DOI] [PubMed] [Google Scholar]
- Kenyon, C., J. Chang, E. Gensch, A. Rudner and R. Tabtiang, 1993. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464. [DOI] [PubMed] [Google Scholar]
- Kim, S. K., J. Lund, M. Kiraly, K. Duke, M. Jiang et al., 2001. A gene expression map for Caenorhabditis elegans. Science 293: 2087–2092. [DOI] [PubMed] [Google Scholar]
- Klass, M., and D. Hirsh, 1976. Non-ageing developmental variant of Caenorhabditis elegans. Nature 260: 523–525. [DOI] [PubMed] [Google Scholar]
- Larsen, P. L., P. S. Albert and D. L. Riddle, 1995. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139: 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, L., and C. S. Hill, 2006. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 17: 41–58. [DOI] [PubMed] [Google Scholar]
- Macdonald, J., Z. Li, W. Su and L. J. Pike, 2006. The membrane proximal disulfides of the EGF receptor extracellular domain are required for high affinity binding and signal transduction but do not play a role in the localization of the receptor to lipid rafts. Biochim. Biophys. Acta 1763: 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, E. A., and J. H. Thomas, 1994. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics 136: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, C. T., S. A. McCarroll, C. I. Bargmann, A. Fraser, R. S. Kamath et al., 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424: 277–283. [DOI] [PubMed] [Google Scholar]
- Ogg, S., S. Paradis, S. Gottlieb, G. I. Patterson, L. Lee et al., 1997. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389: 994–999. [DOI] [PubMed] [Google Scholar]
- Ohkura, K., N. Suzuki, T. Ishihara and I. Katsura, 2003. SDF-9, a protein tyrosine phosphatase-like molecule, regulates the L3/dauer developmental decision through hormonal signaling in C. elegans. Development 130: 3237–3248. [DOI] [PubMed] [Google Scholar]
- Paradis, S., and G. Ruvkun, 1998. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12: 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, G. I., A. Koweek, A. Wong, Y. Liu and G. Ruvkun, 1997. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 11: 2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk, R. H., and J. T. Groenen, 1992. Targeted alterations of the Caenorhabditis elegans genome by transgene instructed DNA double strand break repair following Tc1 excision. EMBO J. 11: 287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, P., C. S. Lim, R. Johnsen, P. S. Albert, D. Pilgrim et al., 1996. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L., and P. S. Albert, 1997. Genetic and environmental regulation of dauer larva development, pp. 739–768 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Romano, G., 2003. The complex biology of the receptor for the insulin-like growth factor-1. Drug News Perspect. 16: 525–531. [DOI] [PubMed] [Google Scholar]
- Swanson, M. M., and D. L. Riddle, 1981. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev. Biol. 84: 27–40. [DOI] [PubMed] [Google Scholar]
- Tewari, M., P. J. Hu, J. S. Ahn, N. Ayivi-Guedehoussou, P. O. Vidalain et al., 2004. Systematic interactome mapping and genetic perturbation analysis of a C. elegans TGF-beta signaling network. Mol. Cell 13: 469–482. [DOI] [PubMed] [Google Scholar]
- Wishart, M. J., and J. E. Dixon, 1998. Gathering STYX: phosphatase-like form predicts functions for unique protein-interaction domains. Trends Biochem. Sci. 23: 301–306. [DOI] [PubMed] [Google Scholar]
- Witherspoon, D. J., and H. M. Robertson, 2003. Neutral evolution of ten types of mariner transposons in the genomes of Caenorhabditis elegans and Caenorhabditis briggsae. J. Mol. Evol. 56: 751–769. [DOI] [PubMed] [Google Scholar]
- Yu, H., and P. L. Larsen, 2001. DAF-16-dependent and independent expression targets of DAF-2 insulin receptor-like pathway in Caenorhabditis elegans include FKBPs. J. Mol. Biol. 314: 1017–1028. [DOI] [PubMed] [Google Scholar]