Abstract
The cho-1 gene in Caenorhabditis elegans encodes a high-affinity plasma-membrane choline transporter believed to be rate limiting for acetylcholine (ACh) synthesis in cholinergic nerve terminals. We found that CHO-1 is expressed in most, but not all cholinergic neurons in C. elegans. cho-1 null mutants are viable and exhibit mild deficits in cholinergic behavior; they are slightly resistant to the acetylcholinesterase inhibitor aldicarb, and they exhibit reduced swimming rates in liquid. cho-1 mutants also fail to sustain swimming behavior; over a 33-min time course, cho-1 mutants slow down or stop swimming, whereas wild-type animals sustain the initial rate of swimming over the duration of the experiment. A functional CHO-1∷GFP fusion protein rescues these cho-1 mutant phenotypes and is enriched at cholinergic synapses. Although cho-1 mutants clearly exhibit defects in cholinergic behaviors, the loss of cho-1 function has surprisingly mild effects on cholinergic neurotransmission. However, reducing endogenous choline synthesis strongly enhances the phenotype of cho-1 mutants, giving rise to a synthetic uncoordinated phenotype. Our results indicate that both choline transport and de novo synthesis provide choline for ACh synthesis in C. elegans cholinergic neurons.
ACETYLCHOLINE (ACh) is a major neurotransmitter in both vertebrate and invertebrate nervous systems. ACh is synthesized in neurons from choline and acetyl-CoA by choline acetyltransferase (ChAT) and is packaged into synaptic vesicles by the vesicular ACh transporter (VAChT). After synaptic release, ACh interacts with receptors on target cells, eliciting a variety of postsynaptic responses. The action of ACh in the synaptic cleft is terminated by acetylcholinesterases (AChEs), which cleave ACh to yield choline and acetate; the choline is taken back up by neurons through high-affinity plasma-membrane choline transporters (CHTs). Choline reuptake through high-affinity transporters is thought to be the major source of choline for ACh synthesis.
The existence of a high-affinity CHT activity in rat brain synaptosomes was first reported more than 30 years ago (Yamamura and Snyder 1972; Haga and Noda 1973). The transport activity is Na+ and Cl− dependent, has a Km for choline of ∼1 μm, and is inhibited by hemicholinium-3 (HC-3; Ki ∼100 nm). Subcellular fractionation and ligand-binding studies (using labeled HC-3) suggested that the transporter is associated with cholinergic terminals, and it is widely believed that the choline transported into the terminal is rate limiting for ACh synthesis (Jope and Jenden 1980; Collier 1988; Bussiere et al. 2001).
The choline transporter was finally cloned and characterized in 2000, with the identification of the Caenorhabditis elegans C48D1.3 (now renamed cho-1) gene product as the CHT protein (Okuda et al. 2000). These investigators also demonstrated that a GFP reporter driven by cho-1 upstream sequences was expressed in (at least some) cholinergic motor neurons. The cho-1 sequence was then used to isolate a rat homolog (termed CHT1), and descriptions of the human, mouse, and Limulus CHT1 homologs soon followed (Apparsundaram et al. 2000, 2001; Okuda and Haga 2000; Wang et al. 2001). The amino acid sequence of the CHT proteins revealed no substantial similarity to previously identified neurotransmitter transporters, but rather to a class of Na+-dependent transporters with substrates such as glucose (SGLT1) and inositol (SMIT1).
CHT knockout mutations in mice result in early neonatal lethality, associated with severe impairment of cholinergic neurotransmission (Ferguson et al. 2004). This observation supports the view that choline uptake through CHT is the major source of choline for ACh synthesis in cholinergic neurons. However, Ferguson et al. (2004) and other studies suggest that at least some ACh is synthesized in the absence of CHT function. In the present study, we show that the C. elegans CHO-1 protein is expressed in virtually all cholinergic neurons and is enriched at synaptic regions. In contrast to the mouse CHT knockout mutants, cho-1 null mutants are viable and relatively coordinated; however, they do exhibit mild deficits in cholinergic behaviors. Finally, we demonstrate that both CHO-1-dependent transport and de novo synthesis of choline are the major contributors to the choline pool used for the synthesis of ACh in C. elegans cholinergic neurons.
MATERIALS AND METHODS
Strains and strain maintenance:
C. elegans was grown on solid medium as described by Brenner (1974), modified by the addition of streptomycin and mycostatin to reduce contamination and by the use of the streptomycin-resistant bacterial strain OP50/1 (Johnson et al. 1988). The cho-1(tm373) mutant was obtained from Shohei Mitani (Tokyo Women's Medical College, Tokyo), and the chtl-1(ok1695) mutant was provided by the C. elegans Gene Knockout Consortium. The unc-104(e1265), pha-1(e2123), and rrf-3(pk1426) mutants were provided by the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis).
Sequencing of mutants:
Mutations were analyzed by amplification of specific cho-1 genomic regions from individual mutant animals (Barstead and Waterston 1989), followed by sequencing of the purified PCR product with nested primers. DNA sequencing was performed at the OMRF DNA Sequencing Core Facility, using oligonucleotide primers obtained from IDT (Coralville, IA).
Reporter constructs and functional fusions:
Plasmids containing the CFP, YFP, or GFP coding sequences were derived from the pPD95.67 plasmid (Miller et al. 1999). cho-1 promoter and functional fusions with GFP were generated using a PCR-fusion approach with overlapping PCR primers (Horton et al. 1989; Hobert 2002).
Transgenic methods:
Transgenic nematodes were obtained by micro-injection of DNA, essentially as described by Mello et al. (1991). Transformation markers included the pBX plasmid (Heinke and Ralf Schnabel, Max-Planck-Institut für Biochemie), which rescues the temperature-sensitive lethality of pha-1(e2123) mutants. We constructed appropriate recipient strains for transformation containing the pha-1(e2123) mutation. Extrachromosomal arrays were integrated by gamma irradiation (Schade et al. 2005).
Behavioral assays:
All behavioral measurements were performed at ∼22°. Swimming rates were measured on hermaphrodites raised at 20° as described previously (Miller et al. 1996). Acute aldicarb-resistance assays were performed as described previously (Lackner et al. 1999; Nurrish et al. 1999), using 2 mm aldicarb. Paralysis was defined as the complete absence of spontaneous and provoked (prodded with a worm pick) locomotion. For chronic aldicarb-resistance assays, we transferred 20 eggs per genotype to plates containing 0.5 mm aldicarb and counted the number of progeny 7 days later. To monitor prolonged swimming behavior, five worms were transferred to M9 buffer (5 ml on a 60-mm NGM-L plate) and allowed to equilibrate for 1 min. The fraction of worms actively swimming was scored at 3-min intervals for a 33-min time course. To score reversal behavior, worms grown at 20° were transferred to room temperature plates with no food. They were allowed to equilibrate for 2 min and then were scored visually for both forward and reverse movements for 10 min using the Etho 1.2.2 program (provided by James H. Thomas, Genome Sciences, University of Washington). Only changes in direction were scored; pauses were disregarded. Statistical significance tests used the Mann–Whitney U-test.
Immunofluorescence staining:
Antibodies used in this study included rabbit (Molecular Probes, Eugene, OR) and chicken (Chemicon, Temecula, CA) polyclonal α-GFP antibodies, and a monoclonal antibody (mAb1403) to UNC-17/VAChT, which has been described previously (Lickteig et al. 2001). Secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Nematodes were stained using a modified freeze-fracture procedure as previously described (Duerr et al. 1999; Mullen et al. 2006).
Microscopy and imaging:
Confocal images were collected on a Leica TCS NT confocal microscope. Low-resolution images were collected with a 40× Plan Fluotar 1.0 NA oil immersion objective, at 1024 × 1024 pixels, with 0.5 μm Z-steps. High-resolution images (insets in Figure 3) were collected with a 63× Plan APO 1.4 NA oil immersion objective, at 1024 × 1024 pixels, with 0.1 μm Z-steps. Images were cropped to size, assembled, and annotated using Adobe Photoshop CS2. Digital manipulations were limited to rotating, resizing (Photoshop Bicubic), and minor levels adjustments. Transmitted light images and videos were collected on an Olympus SZX12 microscope equipped with a Photometrics CoolSNAP camera (Roper Scientific, Duluth, GA) using MetaVue 6.1 software (Universal Imaging, West Chester, PA).
Figure 3.—
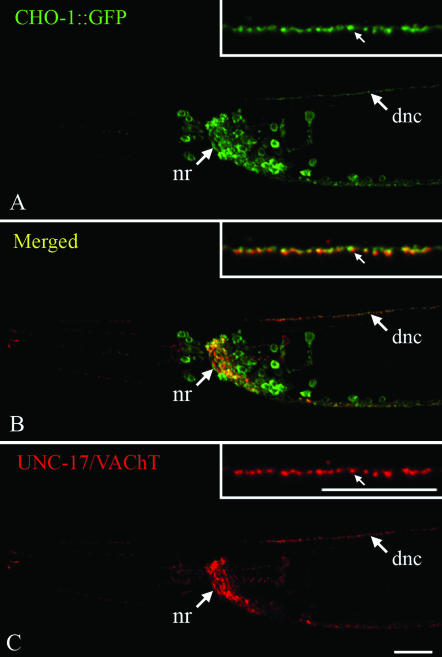
A functional CHO-1∷GFP fusion is localized to cholinergic synapses. Transgenic animals were stained with anti-GFP (green, A and B) and anti-UNC-17/VAChT (red, B and C) antibodies. Head region, anterior is to the left, ventral is down, and the bar is ∼10 μm. Insets are magnified sections of the dorsal nerve cord and correspond to ∼20 μm. The positions of the nerve ring (nr) and dorsal nerve cord (dnc) are indicated. The CHO-1∷GFP fusion is localized to cholinergic synapses and is slightly offset from the pool of VAChT-containing synaptic vesicles.
RNA interference:
Worms were exposed to RNA interference (RNAi) through the feeding method described by Timmons et al. (2001). We constructed a pmt-2 RNAi plasmid (RM#895p) in the pPD129.36 plasmid vector (Timmons and Fire 1998) using the primers described by Palavalli et al. (2006). Bacteria containing this construct or the control plasmid (pPD129.36) were spread on NGM-L plates (without streptomycin) supplemented with isopropyl 1-thio-β-d-galactopyranoside (IPTG) and ampicillin. For chemical rescue with exogenous choline, choline chloride was added to a 30-mm final concentration. L4 hermaphrodites were washed briefly in M9 buffer and then transferred to RNAi plates. Plates were scored 4 days later as described by Palavalli et al. (2006), and behavioral analyses were conducted at this stage. Initially, all RNAi experiments were performed on worms carrying the rrf-3(pk1426) mutation, which results in enhanced sensitivity to RNAi in the nervous system (Simmer et al. 2002). However, we found that equivalent results were observed without this mutation in the background; the results in the present study are from worms with a wild-type genetic background.
RESULTS
The CHO-1 transporter is expressed in most cholinergic neurons:
To examine the expression pattern of the cho-1 gene, we generated two cho-1 transcriptional reporters, with ∼5.2 and ∼7.6 kb of upstream sequence, driving YFP or CFP expression, respectively (Figure 1). The 5.2-kb reporter approximately replicates the reporter described by Okuda et al. (2000), while the 7.6-kb reporter contains additional upstream sequences. These additional upstream sequences include regions that are conserved between C. elegans and the closely related nematode C. briggsae and may represent transcriptional regulatory elements. We generated independent transgenic lines carrying these reporters and compared their expression patterns. We found that both Pcho-1∷GFP fusions are strongly expressed in the nervous system. However, there were some differences between the two reporters; the 7.6-kb reporter was expressed in several head neurons that did not express the 5.2-kb reporter (data not shown).
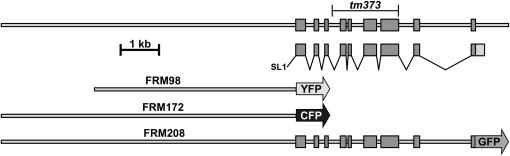
Figure 1.—
Diagram of the cho-1 gene, showing intron–exon structure, and location of the tm373 deletion breakpoints. Also shown are the cho-1 promoter fusions (FRM98 and FRM172) and full-length CHO-1∷GFP functional fusion (FRM208) described in this study.
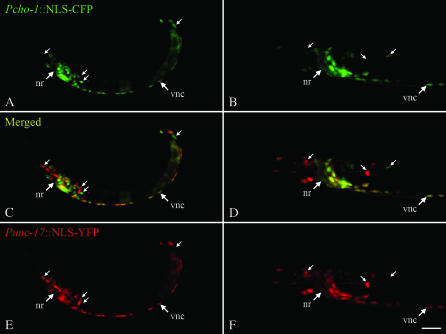
We compared the expression pattern of the 7.6-kb cho-1 reporter with that of a well-characterized cholinergic reporter (Punc-17∷YFP). We found that the 7.6-kb reporter was expressed in most cholinergic neurons, as well as some nonneuronal cells, including the posterior-most three or four intestinal cells (Figure 2). The cho-1 reporter was not expressed in all cholinergic neurons; at least three cholinergic neurons in the head lacked detectable expression (Figure 2). In addition, the cho-1 reporter was expressed in several additional (presumably noncholinergic) neurons in the head and tail. Thus, expression of the CHO-1 transporter is largely, but not exclusively associated with cholinergic neurons. We also noted that the relative expression levels of the two reporters varied greatly between cells (Figure 2).
Figure 2.—
The cho-1 gene is expressed in most cholinergic neurons. Transgenic animals carrying the Pcho-1∷NLS-CFP (green, A–D) and Punc-17∷NLS-YFP (red, C–F) transgenes were imaged on a confocal microscope. An L1 larva is shown in A, C, and E, and the head region of an adult hermaphrodite is shown in B, D, and F. Anterior is to the left, ventral is down, and the bar is ∼10 μm. The positions of the nerve ring (nr) and ventral nerve cord (vnc) are indicated. Arrows indicate some of the cells exclusively expressing either cho-1 or unc-17/cha-1.
The CHO-1 protein is localized to cholinergic synapses:
We generated a CHO-1∷GFP fusion protein (under control of the 7.6-kb cho-1 promoter) to determine the localization of the CHO-1 protein. This fusion protein is functional because it rescues several cho-1 mutant behaviors (see below). Transgenic animals expressing this fusion protein were stained with anti-GFP and anti-UNC-17 (VAChT) antibodies. Confocal microscopy revealed that CHO-1∷GFP is present at most cholinergic synapses, beginning during embryogenesis and persisting throughout development (Figure 3). We also observed CHO-1∷GFP fluorescence in the neuronal cell bodies; we believe that this reflects overexpression of the CHO-1∷GFP transgene. In neuronal processes, the CHO-1∷GFP was localized near cholinergic synaptic vesicles as indicated by anti-UNC-17 staining, but CHO-1 and UNC-17 did not fully colocalize, consistent with a plasma membrane localization of CHO-1 (Figure 3). We thus conclude that a significant fraction of synaptically localized CHO-1 is not physically associated with cholinergic synaptic vesicles.
cho-1 null mutants have moderate deficits in cholinergic function:
To determine the role of CHO-1 in cholinergic neurotransmission, we examined a cho-1 deletion mutation, tm373. The tm373 mutation is associated with a 1695-bp deletion that eliminates more than half of the cho-1 coding sequence (Figure 1); it is almost certainly a null allele. This mutation has been shown to eliminate nearly all high-affinity choline uptake in C. elegans neurons (Matthies et al. 2006). Animals homozygous for this mutation are viable, and their growth and development appear to be normal, although they are slightly long and thin. Since cholinergic neurotransmission is essential for viability in C. elegans (Alfonso et al. 1993, 1994), we conclude that the CHO-1 protein is not essential for cholinergic function.
Because tm373 homozygotes do not exhibit a dramatic mutant phenotype on agar plates, we examined several cholinergic phenotypes and behaviors, including locomotion and response to aldicarb, to determine whether there were subtle defects in cholinergic function. Resistance to the AChE inhibitor aldicarb is usually associated with decreased ACh release (Miller et al. 1996). Aldicarb resistance can be quantified in a variety of ways, including acute assays, in which hypercontraction or paralysis are monitored over time (Lackner et al. 1999; Nurrish et al. 1999), or chronic assays, in which the ability to grow and reproduce is monitored (Miller et al. 1996). We found that cho-1 mutants are just as sensitive as wild-type animals to 2 mm aldicarb in an acute assay (see below). However, cho-1 mutants are somewhat resistant to aldicarb in a chronic exposure paradigm. We transferred 20 eggs per genotype to plates containing 0.5 mm aldicarb and counted the number of progeny 7 days later. Under these conditions, wild-type animals were not capable of growing or reproducing. In contrast, the cho-1 mutant animals grew slowly and reproduced (>100 animals), and the positive control for aldicarb resistance, unc-17(e245), was too numerous to count (>500 animals). Therefore, cho-1 mutants are somewhat resistant to aldicarb, suggesting that they have moderate deficits in ACh synthesis or release.
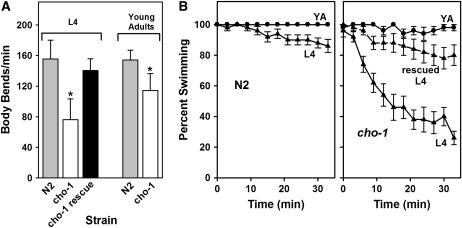
In C. elegans, ACh acts at neuromuscular junctions (NMJs) as an excitatory neurotransmitter; consequently, mutants with defects in ACh synthesis or release exhibit locomotory defects. Therefore, we assayed the locomotory behavior of cho-1 mutants using several movement paradigms. On agar plates, the cho-1 mutants moved slightly more slowly than wild-type animals, but appeared to be normally coordinated (supplemental Videos 1 and 2 at http://www.genetics.org/supplemental/). However, when placed in liquid, the cho-1 animals were clearly less vigorous than wild-type animals, and swam at approximately 50–70% of the wild-type rate (Figure 4A). We noted that these locomotory defects were most pronounced in L4 larvae, suggesting that cho-1 function may be more important at this stage.
Figure 4.—
cho-1 mutants exhibit reduced initial swimming rates and fail to sustain swimming over an extended time. (A) Swimming rates. Data are presented as body bends per minute plus or minus standard deviation for 25 animals. cho-1 L4 larvae were significantly more impaired than were young adults. Expression of the CHO-1∷GFP fusion protein rescued the L4 swimming defect. Values that are significantly different (P < 0.001; Mann–Whitney U-test) from wild type are indicated (*). (B) The percentage of animals actively swimming was scored at 3-min intervals for a 33-min time course. Data are presented as the percentage of animals swimming, plus or minus standard error of the mean, for a total of 50 animals at each time point. cho-1(tm373) L4 larvae exhibited a dramatic decline (fatigue) in swimming rate, whereas the young adults (YA) did not fatigue over the duration of the experiment. Expression of the CHO-1∷GFP fusion protein rescued the L4 fatigue phenotype.
We also monitored the fraction of animals swimming vigorously over a 33-min time course. We found that wild-type worms sustained swimming behavior over the duration of the experiment (Figure 4B), although the fraction of L4 larvae swimming declined slightly (14%) with time. Likewise, cho-1 mutants at most developmental stages (L1, L2, and L3 larvae and young adults) sustained swimming behavior. However, the fraction of cho-1 L4 larvae swimming declined dramatically (∼70%) over the course of the experiment. The CHO-1∷GFP fusion protein described above rescued both the reduced initial swimming rate and failure to sustain swimming behavior (Figure 4), confirming that these phenotypes are specifically due to the cho-1 mutation. These results suggest that cho-1 activity is required to support prolonged vigorous movement and imply that choline reuptake through CHO-1 is more important at certain developmental stages.
cho-1 mutants also exhibit subtle defects in locomotory behaviors when observed on agar plates. We scored reversal frequencies and forward run times for wild-type and cho-1 animals on plates lacking food. We found that cho-1 mutants executed spontaneous reversals far more frequently than wild-type animals. The mean forward run time was 21.5 ± 7.7 sec for wild-type and 9.9 ± 1.7 sec for cho-1 mutants. The increase in reversal frequency resulted in a significant increase in forward runs of short duration (arbitrarily defined as runs <5 sec; wild type, 25.1 ± 13.9%; cho-1, 43.8 ± 9.9%). These results suggest that cholinergic signaling may regulate reversal frequency.
Blocking choline biosynthesis enhances the cho-1 phenotype:
There is considerable evidence from other organisms that choline uptake through CHT proteins is essential for sustaining cholinergic transmission (Collier 1988; Ferguson et al. 2004). The relatively mild phenotype of cho-1 mutants was therefore surprising, since cholinergic neurotransmission is essential for viability in C. elegans (Alfonso et al. 1993, 1994). Clearly, in the absence of cho-1 function, choline for ACh synthesis must be supplied in other ways, either through other transporters, or by de novo synthesis. We examined both of these possibilities.
Recently, Palavalli et al. (2006) demonstrated that a major pathway for phosphocholine biosynthesis in C. elegans is through the methylation of phosphoethanolamine. Conversion of phosphocholine to choline by dephosphorylation could potentially provide a source of choline for ACh synthesis. Through RNA interference, Palavalli et al. (2006) demonstrated that the C. elegans phosphoethanolamine methyltransferase gene (pmt-2) is essential for growth and development and that supplementation with choline can bypass the requirement for this enzyme.
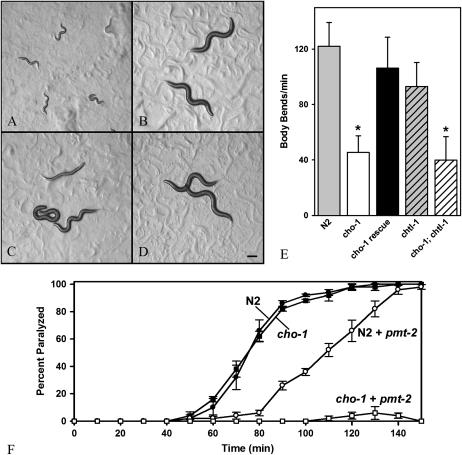
We took advantage of these observations to examine the contributions of choline synthesis and reuptake to ACh synthesis. We generated an RNAi construct as described by Palavalli et al. (2006) to knock down synthesis of choline through the pmt-2-dependent pathway. L4 hermaphrodites were exposed to RNAi in the presence or absence of exogenous choline (30 mm). As reported by Palavalli et al., in the absence of supplemental choline, knockdown of pmt-2 function resulted in a fully penetrant early-larval arrest phenotype in the F1 progeny (Figure 5A). This developmental arrest was fully rescued by providing supplemental choline (Figure 5B and supplemental Video 3 at http://www.genetics.org/supplemental/). In the absence of cho-1 function, supplemental choline rescued viability of the F1 progeny, but the worms were highly uncoordinated (Figure 5C and supplemental Video 4) and exhibited a phenotype similar to that of cholinergic mutants such as cha-1 and unc-17 (Rand and Russell 1984). The treated cho-1 animals were significantly defective in locomotory behavior relative to control animals in swimming assays (Figure 5E). In addition, these animals were also strongly resistant to aldicarb in an acute assay (Figure 5F), indicating that ACh release was significantly reduced. The CHO-1∷GFP fusion protein rescued the synthetic uncoordinated phenotype (Figure 5, D and E), demonstrating that this phenotype is associated specifically with the cho-1 mutation. These results suggest that choline synthesis through a pmt-2-dependent pathway is a significant contributor to the pool of intracellular choline utilized for synthesis of ACh.
Figure 5.—
RNAi knockdown of choline biosynthesis enhances the cho-1 phenotype. (A–D) Wild-type (N2) and cho-1(tm373) animals were exposed to pmt-2 RNAi, with or without supplemental choline. Bar, ∼0.1 mm. In the absence of supplemental choline, F1 wild-type animals arrested development as L1 or L2 larvae (A). This larval-arrest phenotype was rescued by addition of supplemental choline (B; see also supplemental Video 3 at http://www.genetics.org/supplemental/). cho-1 mutants were viable in the presence of pmt-2 RNAi with supplemental choline, but were highly uncoordinated as evidenced by body posture (C; see also supplemental Video 4) and analysis of swimming behavior (E). This phenotype was fully rescued by expression of a CHO-1∷GFP functional fusion protein (D and E). (E) Swimming rates of wild-type and mutant animals (exposed to pmt-2 RNAi with supplemental choline). Data are presented as body bends per minute plus or minus standard deviation for 10 animals. Values that are significantly different (P <0.001; Mann–Whitney U-test) from wild type are indicated (*). (F) Paralysis of cho-1(tm373) and wild-type animals was monitored over a 2.5-hr time course on plates containing 2 mm aldicarb. Data are presented as the percentage of animals paralyzed plus or minus standard error of the mean for a total of 50 animals at each time point. Untreated cho-1 and wild-type animals were grown and assayed on HT115(D3) bacteria containing the control (pPD129.36) RNAi plasmid. Treated cho-1 and wild-type animals were grown and assayed on HT115(D3) bacteria containing the pmt-2 (RM#895p) RNAi plasmid and media containing 30 mm choline. Control experiments indicated that 30 mm choline (in the absence of pmt-2 RNAi) had minimal effect on the aldicarb response of wild-type and cho-1 animals. Untreated cho-1 mutants were not resistant to acute aldicarb exposure, whereas cho-1 mutants exposed to pmt-2 RNAi and given supplemental choline were strongly resistant.
The CHO-1 transporter is the major pathway for choline reuptake in cholinergic neurons:
Other vertebrate proteins known to transport choline include the family of choline transporter-like (CTL) proteins. C. elegans has a single CTL homolog, encoded by the chtl-1 gene. We obtained a deletion mutation for the chtl-1 gene from the Gene Knockout Consortium; chtl-1(ok1695) is associated with an 813-bp deletion and is likely to be a null allele. chtl-1 mutants do not exhibit obvious mutant phenotypes; crawling and swimming behaviors were not significantly different from those of wild-type animals (data not shown). We asked whether a null mutation in the chtl-1 gene could block chemical rescue of the pmt-2 RNAi phenotype with supplemental choline. We found that the chtl-1 gene was not required for chemical rescue of the pmt-2 RNAi phenotype; supplemental choline fully rescued growth and viability. The rescued worms did exhibit a modest reduction in swimming relative to wild-type animals (Figure 5E). However, the chtl-1 mutation did not enhance the cho-1 mutant phenotype in this assay: cho-1(tm373); chtl-1(ok1695) double mutants exhibited swimming rates comparable to those of cho-1 single mutants (Figure 5E). Overall, our results suggest that CHTL-1 does not contribute significantly to the uptake of choline for ACh synthesis in neurons. In addition, choline uptake through this transporter is clearly not a major source of choline for phospholipid synthesis in nonneuronal cells.
DISCUSSION
The CHO-1 transporter is expressed in cholinergic neurons and is enriched in synaptic regions:
We report here that the C. elegans high-affinity choline transporter CHO-1 is expressed chiefly in cholinergic neurons (see also Okuda et al. 2000; Matthies et al. 2006). Similarly, CHT proteins in rats, mice, and humans are expressed in most cholinergic neurons (Misawa et al. 2001; Ferguson et al. 2003), consistent with their presumed primary role in providing choline for ACh synthesis. In the present study, we also noted expression of CHO-1 in several (apparently) noncholinergic neurons, as well as in the intestine. In contrast to a previous report (Matthies et al. 2006), we found that CHO-1 is not expressed in all cholinergic neurons; several cholinergic neurons in the head do not appear to express CHO-1 (Figure 2). However, since previous studies (Okuda et al. 2000; Matthies et al. 2006) did not compare the expression of CHO-1 with that of a well-characterized cholinergic marker (such as unc-17/VAChT), it is difficult to assess the extent of conflict between our results and theirs. Sequence analysis of the cho-1 and unc-17 promoters indicates that although there are some conserved elements, there are significant differences between the two promoters, consistent with the observed differences in expression.
Like mammalian CHT proteins, CHO-1 is significantly enriched in synaptic regions (Figure 3). Similar results were reported recently by Matthies et al. (2006). In mice, a significant fraction of CHT protein appears to be associated with cholinergic synaptic vesicles (Ferguson et al. 2003). This observation has led to an appealing model of CHT trafficking and function, whereby the process of releasing vesicular ACh simultaneously delivers CHT to the plasma membrane (Ferguson et al. 2003; reviewed in Ferguson and Blakely 2004). We found that the distribution of CHO-1 only partially overlaps with that of VAChT (Figure 3), suggesting that a significant fraction of CHO-1 is not present on cholinergic synaptic vesicles. Careful examination of the images presented in the Matthies et al. (2006) study also supports this conclusion. Axonal transport of CHO-1 to synapses is dependent on UNC-104 (Matthies et al. 2006 and our unpublished results), a kinesin-like motor protein required for axonal transport of synaptic vesicle proteins (Hall and Hedgecock 1991; Otsuka et al. 1991). This observation implies that CHO-1 is associated with synaptic vesicles or perhaps another type of UNC-104-dependent vesicle. However, if in fact CHO-1 is associated with synaptic vesicle precursors during axonal transport, then our results suggest that a significant fraction of CHO-1 is selectively redistributed from nascent synaptic vesicles after reaching the synapse. As a cautionary note, both this and the Matthies et al. (2006) study used transgenic expression of a CHO-1∷GFP fusion protein to examine CHO-1 localization. Overexpression of the CHO-1∷GFP could potentially lead to some ectopic localization.
cho-1 null mutants exhibit relatively mild cholinergic deficits:
The loss of cho-1 function in C. elegans does not result in a dramatic mutant phenotype; cho-1 null mutants are viable and move in a coordinated manner (supplemental Video 2 at http://www.genetics.org/supplemental/). However, cho-1 mutants do exhibit several mild cholinergic defects: they are moderately resistant to aldicarb in a chronic-exposure paradigm, swim more slowly than wild-type animals, and at least at certain developmental stages are unable to sustain swimming behavior more than several minutes. The inability of cho-1 mutants to sustain swimming behavior was also noted by Matthies et al. (2006), although these investigators used a different experimental approach and did not report behavioral differences between developmental stages. We interpret our results to mean that there is sufficient choline from low-affinity uptake and de novo synthesis to support a significant level of ACh synthesis in the absence of high-affinity uptake activity and that this level of ACh synthesis is sufficient for locomotion in a calm environment. However, this level of ACh is not adequate to support the increased demands of vigorous swimming, and the cho-1 motor neurons appear to become depleted of neurotransmitter.
cho-1 mutants also exhibited more subtle changes in crawling behavior; in the absence of food, cho-1 mutants spontaneously reversed direction more frequently than wild-type animals and forward run times were considerably reduced. Such defects have not been noted previously in C. elegans cholinergic mutants, perhaps because the severe locomotory defects observed in most cholinergic mutants obscure more subtle behavioral deficits. Although cho-1 mutants exhibit relatively coordinated movements in simple crawling or swimming assays, cholinergic output may be reduced to the point that more complex movement behaviors are affected. Changes in spontaneous reversal frequency are also observed in mutants with defects in glutamatergic or dopaminergic signaling (Hills et al. 2004). Our observations on cho-1 mutants suggest that cholinergic signaling may also regulate reversal frequency.
The mild phenotype of cho-1 mutants may reflect organism-specific differences in ACh requirements:
The mild phenotypes of cho-1 null mutants are somewhat surprising since null mutations in other cholinergic genes in C. elegans, such as choline acetyltransferase (cha-1) or VAChT (unc-17), result in early larval lethality (Alfonso et al. 1993, 1994). Clearly, cho-1 function is not essential for cholinergic signaling in C. elegans. In contrast, CHT function is essential in mammals: CHT −/− mice die shortly after birth, and electrophysiological analyses indicate that the pool of releasable ACh is significantly reduced (Ferguson et al. 2004). However, CHT −/− mice do not exhibit the flaccid paralysis of newborn ChAT −/− mice, indicating that a limited amount of ACh is synthesized in the absence of CHT (Ferguson et al. 2004). Furthermore, several researchers have reported that a small fraction of ACh synthesis is independent of HC-3-sensitive choline uptake (Birks and Macintosh 1957; Guyenet et al. 1973).
We believe that some of the phenotypic differences between C. elegans and mouse CHT knockouts reflect organism-specific differences in ACh requirements. Null mutations in several key synaptic genes, including synaptotagmin 1, are lethal in mice, yet they do not result in lethality in C. elegans (Nonet et al. 1993), despite the fact that these mutations have the same physiological consequences as the analogous mouse knockouts (Geppert et al. 1994). The limited amount of ACh (∼20%) that is synthesized in the absence of CHT in mice would probably support viability, and perhaps even coordinated movement in C. elegans. In addition, the relative contributions of the reuptake and de novo synthesis pathways to the pool of choline used for ACh synthesis may differ between the two organisms, a possibility that we discuss in detail below.
Choline synthesis through the phosphoethanolamine methyltransferase-dependent pathway contributes to ACh biosynthesis in C. elegans:
Choline for ACh biosynthesis can potentially be supplied through three pathways: (1) de novo synthesis, (2) uptake of free choline by low- and high-affinity transporters, and (3) turnover of membrane phospholipids. Assessing the relative contributions of these pathways from the literature is somewhat difficult. Observations on the mouse CHT knockout suggest that choline uptake through the high-affinity transporter is critical for the synthesis of synaptically releasable pools of ACh (Ferguson et al. 2004). However, as noted earlier, researchers have also reported that a small, but significant fraction (∼20%) of ACh synthesis is independent of HC-3-sensitive choline uptake (Birks and Macintosh 1957; Guyenet et al. 1973). Other researchers have demonstrated that neuroendocrine cells are capable of synthesizing and releasing ACh in the absence of detectable CHT expression (Bauerfeind et al. 1993; Ferguson et al. 2003). These observations are generally attributed to the activity of low-affinity choline transporters (Ferguson et al. 2004). However, Blusztajn et al. (1987), using a neuroblastoma cell line, demonstrated that ACh can also be synthesized from choline liberated from membrane phosphatidylcholine by phospholipase D. In the present study, we have shown that reducing de novo choline synthesis through the phosphoethanolamine methyltransferase-dependent pathway enhances the phenotype of cho-1 mutants, giving rise to a synthetic uncoordinated phenotype. This result suggests that de novo synthesis of choline contributes to ACh synthesis in C. elegans; we believe that this is the first demonstration that de novo choline synthesis is important for ACh production in any organism.
Among different organisms, two metabolic pathways have been described for the biosynthesis of choline. In yeast and mammals, phosphatidylserine is converted to phosphatidylethanolamine, which is then converted to phosphatidylcholine through the addition of three methyl groups (CDP-diacylglycerol pathway). Phosphatidylcholine may then be converted to choline through the action of phospholipase D (or other phospholipases). In other organisms, including plants, some protozoans, and nematodes (Pessi et al. 2004; Palavalli et al. 2006), phosphoethanolamine is converted to phosphocholine through the addition of three methyl groups (phosphoethanolamine methyltransferase-dependent pathway). Phosphocholine may then be converted to choline through the action of cellular phosphatases. Based on our analysis of available genome sequences, this pathway is also present in sea urchins, amphibians, and fish, but not in birds or mammals. Therefore, it appears that this pathway has been selectively lost in some organisms, including humans, although it is possible that the activities are present, but the enzymes themselves are not sufficiently similar to be detected through homology searches.
A survey of the literature suggests that cholinergic neurons in the mammalian CNS do not obtain choline through de novo synthesis. Enzymes in the CDP-diacylglycerol pathway, such as phosphatidylethanolamine N-methyltransferase, which converts phosphatidylethanolamine to phosphatidylcholine, are strongly expressed in the liver, but not in neurons (Vance and Ridgway 1988; Shields et al. 2001). In addition, sympathetic neurons cultured in choline-deficient media exhibit marked defects in axonal growth (Vance et al. 1995), suggesting that endogenous synthesis of choline is inadequate to meet choline requirements for neuronal phospholipid synthesis and (presumably) ACh synthesis. Therefore, it appears that de novo choline synthesis is a significant contributor to the pool of choline utilized for synthesis of ACh in cholinergic neurons in nematodes, but not mammals.
The potential role for other choline transporters in supplying choline for ACh synthesis:
Palavalli et al. (2006) demonstrated that eliminating choline biosynthesis through the pmt-2-dependent pathway results in early larval lethality in C. elegans. This lethality can be rescued chemically by providing supplemental choline. As a side note, we found that, under our conditions, the chemically rescued animals were moderately resistant to aldicarb (Figure 5F), suggesting that exogenous choline does not completely compensate for the absence of endogenous synthesis. Using this paradigm, we have shown that blocking choline uptake through the CHO-1 transporter, in the absence of choline synthesis, results in a viable, but highly uncoordinated phenotype (Figure 5C and supplemental Video 4 at http://www.genetics.org/supplemental/). These animals also exhibited enhanced resistance to the acetylcholinesterase inhibiter aldicarb (Figure 5F), indicating that ACh release was greatly reduced. However, there is clearly some residual ACh synthesis (and thus some source of choline) in these animals since mutants with severe defects in cholinergic neurotransmission are lethal. This residual choline might reflect incomplete knockdown of the pmt-2 gene by RNAi, phospholipid turnover via phospholipase D, or uptake of choline through low-affinity transporters. Matthies et al. (2006) reported the presence of a low-affinity choline transport activity in C. elegans cholinergic neurons and suggested that transporters related to the mammalian CTL and OCT proteins might be responsible for this activity. We have excluded the worm CTL homolog, encoded by the chtl-1 gene, as a major contributor to this low-affinity transport activity, both in neuronal and nonneuronal cells. However, there are other potential choline transporters, including members of the organic cation transporter (OCT) family, which could be playing a role. Although the C. elegans oct-1 gene does not appear to be a significant contributor to this low-affinity transport activity (our unpublished results), further studies on other members of the OCT family could clarify their potential roles in choline uptake.
Taken together, our results indicate that the C. elegans CHT protein, like its mammalian homolog, is expressed in almost all cholinergic neurons. In contrast to mice, however, eliminating CHT function in C. elegans does not result in lethality nor does it severely compromise cholinergic neurotransmission. These differences appear to be due, at least in part, to the contribution of de novo choline synthesis to the pool of choline utilized for ACh synthesis in C. elegans. By reducing de novo choline synthesis in a cho-1 mutant background, we revealed a strong synthetic phenotype. In effect, we have created a situation in which choline transport through CHO-1 becomes essential for cholinergic function in C. elegans. This finding will greatly simplify future mutant screens or structure-function studies on the CHT protein in C. elegans.
Acknowledgments
We are grateful to Steve Fields, Ken Miller, Shawn Lockery, and Carl Johnson for useful suggestions and insights; to Jim Henthorn for assistance with confocal imaging; and to Mary Wikswo and Amber Sherrill for technical assistance. Some strains were obtained from the Caenorhabditis Genetics Center, which is supported by the National Center for Research Resources. These studies were supported by grant GM038679 from the National Institute of General Medical Sciences.
References
- Alfonso, A., K. Grundahl, J. S. Duerr, H.-P. Han and J. B. Rand, 1993. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261: 617–619. [DOI] [PubMed] [Google Scholar]
- Alfonso, A., K. Grundahl, J. R. McManus and J. B. Rand, 1994. Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J. Neurosci. 14: 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparsundaram, S., S. M. Ferguson, A. L. George, Jr. and R. D. Blakely, 2000. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem. Biophys. Res. Commun. 276: 862–867. [DOI] [PubMed] [Google Scholar]
- Apparsundaram, S., S. M. Ferguson and R. D. Blakely, 2001. Molecular cloning and characterization of a murine hemicholinium-3-sensitive choline transporter. Biochem. Soc. Trans. 29: 711–716. [DOI] [PubMed] [Google Scholar]
- Barstead, R. J., and R. H. Waterston, 1989. The basal component of the nematode dense-body is vinculin. J. Biol. Chem. 264: 10177–10185. [PubMed] [Google Scholar]
- Bauerfeind, R., A. Regnier-Vigouroux, T. Flatmark and W. B. Huttner, 1993. Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron 11: 105–121. [DOI] [PubMed] [Google Scholar]
- Birks, R. I., and F. C. Macintosh, 1957. Acetylcholine metabolism at nerve-endings. Br. Med. Bull. 13: 157–161. [DOI] [PubMed] [Google Scholar]
- Blusztajn, J. K., M. Liscovitch and U. I. Richardson, 1987. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. USA 84: 5474–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere, M., J. E. Vance, R. B. Campenot and D. E. Vance, 2001. Compartmentalization of choline and acetylcholine metabolism in cultured sympathetic neurons. J. Biochem. 130: 561–568. [DOI] [PubMed] [Google Scholar]
- Collier, B., 1988. About the coupling of acetylcholine hydrolysis and choline uptake at cholinergic nerve terminals. J. Neurochem. 50: 323–324. [DOI] [PubMed] [Google Scholar]
- Duerr, J. S., D. L. Frisby, J. Gaskin, A. Duke, K. Asermely et al., 1999. The cat-1 gene of C. elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J. Neurosci. 19: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, S. M., and R. D. Blakely, 2004. The choline transporter resurfaces: New roles for synaptic vesicles? Mol. Interv. 4: 22–37. [DOI] [PubMed] [Google Scholar]
- Ferguson, S. M., V. Savchenko, S. Apparsundaram, M. Zwick, J. Wright et al., 2003. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J. Neurosci. 23: 9697–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, S. M., M. Bazalakova, V. Savchenko, J. C. Tapia, J. Wright et al., 2004. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl. Acad. Sci. USA 101: 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert, M., Y. Goda, R. E. Hammer, C. Li, T. W. Rosahl et al., 1994. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79: 717–727. [DOI] [PubMed] [Google Scholar]
- Guyenet, P., P. Lefresne, J. Rossier, J. C. Beaujouan and J. Glowinski, 1973. Inhibition by hemicholinium-3 of [14C] acetylcholine synthesis and [3H] choline high-affinity uptake in rat striatal synaptosomes. Mol. Pharmacol. 9: 630–639. [PubMed] [Google Scholar]
- Haga, T., and H. Noda, 1973. Choline uptake systems of rat brain synaptosomes. Biochim. Biophys. Acta 291: 564–575. [DOI] [PubMed] [Google Scholar]
- Hall, D. H., and E. M. Hedgecock, 1991. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847. [DOI] [PubMed] [Google Scholar]
- Hills, T., P. J. Brockie and A. V. Maricq, 2004. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 24: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert, O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen and L. R. Pease, 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77: 61–68. [DOI] [PubMed] [Google Scholar]
- Johnson, C. D., J. B. Rand, R. K. Herman, B. D. Stern and R. L. Russell, 1988. The acetylcholinesterase genes of C. elegans: identification of a third gene (ace-3) and mosaic mapping of a synthetic lethal phenotype. Neuron 1: 165–173. [DOI] [PubMed] [Google Scholar]
- Jope, R. S., and D. J. Jenden, 1980. The utilization of choline and acetyl coenzyme A for the synthesis of acetylcholine. J. Neurochem. 35: 318–325. [DOI] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqα and EGL-8 PLCβ: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346. [DOI] [PubMed] [Google Scholar]
- Lickteig, K. M., J. S. Duerr, D. L. Frisby, D. H. Hall, J. B. Rand et al., 2001. Regulation of neurotransmitter vesicles by the homeodomain protein UNC-4 and its transcriptional corepressor UNC-37/Groucho in Caenorhabditis elegans cholinergic motor neurons. J. Neurosci. 21: 2001–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies, D. S., P. A. Fleming, D. M. Wilkes and R. D. Blakely, 2006. The Caenorhabditis elegans choline transporter CHO-1 sustains acetylcholine synthesis and motor function in an activity-dependent manner. J. Neurosci. 26: 6200–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, D. M., N. S. Desai, D. C. Hardin, D. W. Piston, G. H. Patterson et al., 1999. Two-color GFP expression system for C. elegans. Biotechniques 26: 914–921. [DOI] [PubMed] [Google Scholar]
- Miller, K. G., A. Alfonso, M. Nguyen, J. A. Crowell, C. D. Johnson et al., 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. USA 93: 12593–12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa, H., K. Nakata, J. Matsuura, M. Nagao, T. Okuda et al., 2001. Distribution of the high-affinity choline transporter in the central nervous system of the rat. Neuroscience 105: 87–98. [DOI] [PubMed] [Google Scholar]
- Mullen, G. P., E. A. Mathews, P. Saxena, S. D. Fields, J. R. McManus et al., 2006. The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABA. Mol. Biol. Cell 17: 3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet, M. L., K. Grundahl, B. J. Meyer and J. B. Rand, 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73: 1291–1305. [DOI] [PubMed] [Google Scholar]
- Nurrish, S., L. Ségalat and J. M. Kaplan, 1999. Serotonin inhibition of synaptic transmission: Gαo decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242. [DOI] [PubMed] [Google Scholar]
- Okuda, T., and T. Haga, 2000. Functional characterization of the human high-affinity choline transporter. FEBS Lett. 484: 92–97. [DOI] [PubMed] [Google Scholar]
- Okuda, T., T. Haga, Y. Kanai, H. Endou, T. Ishihara et al., 2000. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 3: 120–125. [DOI] [PubMed] [Google Scholar]
- Otsuka, A. J., A. Jeyaprakash, J. Garcia-Añoveros, L. Z. Tang, G. Fisk et al., 1991. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6: 113–122. [DOI] [PubMed] [Google Scholar]
- Palavalli, L. H., K. M. Brendza, W. Haakenson, R. E. Cahoon, M. McLaird et al., 2006. Defining the role of phosphomethylethanolamine N-methyltransferase from Caenorhabditis elegans in phosphocholine biosynthesis by biochemical and kinetic analysis. Biochemistry 45: 6056–6065. [DOI] [PubMed] [Google Scholar]
- Pessi, G., G. Kociubinski and C. B. Mamoun, 2004. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc. Natl. Acad. Sci. USA 101: 6206–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, J. B., and R. L. Russell, 1984. Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 106: 227–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade, M. A., N. K. Reynolds, C. M. Dollins and K. G. Miller, 2005. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the Gαs pathway and define a third major branch of the synaptic signaling network. Genetics 169: 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, D. J., L. B. Agellon and D. E. Vance, 2001. Structure, expression profile and alternative processing of the human phosphatidylethanolamine N-methyltransferase (PEMT) gene. Biochim. Biophys. Acta 1532: 105–114. [DOI] [PubMed] [Google Scholar]
- Simmer, F., M. Tijsterman, S. Parrish, S. P. Koushika, M. L. Nonet et al., 2002. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 12: 1317–1319. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and A. Fire, 1998. Specific interference by ingested dsRNA. Nature 395: 854. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. [DOI] [PubMed] [Google Scholar]
- Vance, D. E., and N. D. Ridgway, 1988. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 27: 61–79. [DOI] [PubMed] [Google Scholar]
- Vance, J. E., E. P. De Chaves, R. B. Campenot and D. E. Vance, 1995. Role of axons in membrane phospholipid synthesis in rat sympathetic neurons. Neurobiol. Aging 16: 493–498. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Z. Cao, R. F. Newkirk, M. T. Ivy and J. G. Townsel, 2001. Molecular cloning of a cDNA for a putative choline co-transporter from Limulus CNS. Gene 268: 123–131. [DOI] [PubMed] [Google Scholar]
- Yamamura, H. I., and S. H. Snyder, 1972. Choline: high-affinity uptake by rat brain synaptosomes. Science 178: 626–628. [DOI] [PubMed] [Google Scholar]