Abstract
Genetic background and developmental stage influence the function of some disease resistance (R) genes. The molecular mechanisms of these modifications remain elusive. Our results show that the two factors are associated with the expression of the R gene in rice Xa3 (also known as Xa26)-mediated resistance to Xanthomonas oryzae pv. oryzae (Xoo), which in turn influences the expression of defense-responsive genes. The background of japonica rice, one of the two major subspecies of Asian cultivated rice, facilitates the function of Xa3 more than the background of indica rice, another rice subspecies. Xa3 expression gradually increases from early seedling stage to adult stage. Japonica plants carrying Xa3 regulated by the native promoter showed an enlarged resistance spectrum (i.e., resistance to more Xoo races), an increased resistance level (i.e., further reduced lesion length), and whole-growth-stage resistance compared to the indica rice; this enhanced resistance was associated with an increased expression of Xa3 throughout the growth stages in the japonica plants, which resulted in enhanced expression of defense-responsive genes. Overexpressing Xa3 with a constitutive strong promoter further enhanced rice resistance due to further increased Xa3 transcripts in both indica and japonica backgrounds, whereas regulating Xa3 with a pathogen-induced weak promoter impaired rice resistance.
PLANT disease resistance (R) genes are an important source of plant immunity. The encoding products of R genes recognize or guard against specific pathogen effectors and trigger signal transduction cascades that lead to rapid disease resistance in the host plants (Dangl and Jones 2001; Belkhadir et al. 2004). Since each R protein can only directly or indirectly recognize limited types of pathogen effectors, R genes are characterized to mediate race-specific resistance. The same plant species carrying different R genes frequently has a different resistance spectrum to different pathogen species or the same pathogen species but different races. A large number of R proteins have been identified as recognizing different pathogens, including bacteria, fungi, viruses, oomycetes, and nematodes, from diverse plant species; most of the characterized proteins contain a leucine-rich repeat (LRR) domain (Martin et al. 2003). It is generally accepted that the LRR domain of the LRR-containing R proteins is the major contributor of pathogen recognition specificity (Dangl and Jones 2001). A few studies have revealed that non-LRR regions, such as the Toll/interleukin-1 receptor homology region and the region between signal peptide and LRR domain of some R proteins, are also involved in pathogen resistance specificity (Ellis et al. 1999; Luck et al. 2000; Van Der Hoorn et al. 2001).
Although the amino acid sequence of R proteins is an important determinant of pathogen resistance specificity, limited information has shown that other host factors are also required for pathogen recognition in some R gene-mediated disease resistances. Host genetic background is a factor that influences the function of R genes. The rice Xa26 gene conferring resistance to Xanthomonas oryzae pv. oryzae (Xoo), which causes bacterial blight, the most devastating plant bacterial disease worldwide, is one example. Asian-cultivated rice (Oryza sativa L.) consists of two major groups, which are known by the subspecies names indica and japonica. Transgenic plants carrying Xa26 in the background of japonica variety Mudanjiang 8 showed increased resistance to five Xoo strains and enhanced resistance to another three Xoo strains as compared to the gene donor of indica variety Minghui 63 (Sun et al. 2004). In addition, different indica backgrounds also influence the function of R genes. Minghui 63 carries another bacterial blight resistance gene, Xa25(t), in addition to Xa26 (Chen et al. 2002). Another indica rice line IRBB3 is well known to carry only one R gene, Xa3, for Xoo resistance (Ogawa et al. 1991). Our studies have demonstrated that Xa3 and Xa26 are the same gene, with identical sequences in the coding region and only one nucleotide substitution occurring at 475 bp upstream of the translation initiation site (Sun et al. 2004; Xiang et al. 2006). Thus this gene is named Xa3. However, IRBB3 showed better resistance to different Xoo strains than Minghui 63 (Sun et al. 2004), although this difference may be partly caused by different quantitative trait loci for disease resistance in the two genetic backgrounds. Furthermore, the function of an allele of the Arabidopsis R gene RPS2 is influenced by genetic background, and the LRR domain determines the effectiveness of the interaction between RPS2 and other host factors in RPS2-mediated resistance (Banerjee et al. 2001).
The developmental stage of the host is another factor that influences the function of R genes. The activity of rice bacterial blight resistance gene Xa21 is developmentally controlled. Xa21-mediated resistance increases progressively from the susceptible juvenile stage to full resistance at the later adult stage (Century et al. 1999). Several other rice R genes conferring resistance to Xoo also mediate full disease resistance only in the adult stage (Zhang and Mew 1985; Mew 1987; Goel and Gupta 1990; Ogawa 1993). Developmentally controlled disease resistance has also been observed in other plant-pathogen systems. The Cf-9B is a family member of the tomato Cf-9 gene, conferring resistance to Cladosporium fulvum; Cf-9B mediates weaker resistance than Cf-9 and protects only mature plants from infection (Panter et al. 2002).
Although different host factors can modify the function of R genes, the molecular mechanisms of these modifications remain elusive. Here we report that the expression pattern of rice Xa3, encoding LRR receptor kinase type of protein, is associated with its variant resistant activity in different genetic backgrounds and different developmental stages. A higher expression level of Xa3 results in a wider resistance spectrum, a strong resistance level, and whole-growth-stage resistance. The explanation of the dosage-dependent resistance conferred by Xa3 is discussed. Xa3 may be used as a tool to unravel the molecular mechanisms of R protein function in genetic background-dependent and developmental stage-dependent disease resistance. In addition, Xa3-overexpressing plants showed no remarkable morphologic and developmental difference from wild type, implicating the gene's value in breeding programs.
MATERIALS AND METHODS
Plant transformation:
The overexpression construct carrying PUbi:Xa3 was made by inserting the genomic fragment of the Xa3 coding region amplified using primers MRKbR and MRKbF (supplemental Table 1 at http://www.genetics.org/supplemental/) into vector pU1301 (Qiu et al. 2007) (supplemental Figure 1A). The construct carrying PWRKY13:Xa3 was made by inserting the Xa3 coding region amplified using primers MRKbR and MRKbF into vector pI1381 (supplemental Figure 1B). The pI1381 was modified by insertion of a 728-bp rice WRKY13 gene promoter located at −691 to +37 of WRKY13 (Qiu et al. 2007) into the multiple cloning sites of vector pCAMBIA1381. The construct (MKb) carrying PXa3:Xa3 was the same as used previously (supplemental Figure 1C; Sun et al. 2004). Agrobacterium-mediated transformation was performed according to the protocol of Lin and Zhang (2005).
Pathogen inoculation:
To evaluate bacterial blight disease, plants were inoculated with Xoo strains at the seedling or booting stage, as described previously (Sun et al. 2004). Z173 is a Chinese strain. PXO61, PXO86, PXO79, PXO71, PXO99, PXO145, and PXO280 are strains representing Philippine races 1, 2, 3, 4, 6, 7, and 8, respectively. T7174, T7147, and T7133 are Japanese strains. Disease was scored by measuring the percentage of lesion area (lesion length/leaf length) at 2–3 weeks after inoculation. Mock-inoculated (control) plants were treated under the same conditions, except that pathogen suspension was replaced with water.
Reverse transcription–quantitative PCR analysis:
Reverse transcription–quantitative PCR analysis (RT–qPCR) was conducted as described by Wen et al. (2003). Quantitative PCR was performed using primers RealF and Real2R for the plants in the Zhonghua 11 background and using primers RKb3F and RealR for the plants in the backgrounds of Mudanjiang 8, 02428, Minghui 63, or IRBB3 (supplemental Table 1 at http://www.genetics.org/supplemental/) and the ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. The expression level of actin was used to standardize the RNA sample for each RT–qPCR. The qPCR reaction was in a 25-μl volume containing 1 μl of diluted reverse transcription product, 12.5 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems), and 0.32 μm of each primer. For each analysis, RT–qPCR assays were repeated at least twice with each repetition having three replicates; similar results were obtained in repeated experiments.
RESULTS
Japonica background facilitates the function of Xa3:
Our previous study has shown that Xa3 (also known as Xa26) mediates race-specific resistance to Xoo; transgenic rice plants carrying Xa3 in the genetic background of japonica rice variety Mudanjiang 8 showed enhanced resistance as compared with a Xa3 donor, indica rice variety Minghui 63 (Sun et al. 2004). To explore whether this is a general phenomenon, we transferred the Xa3 gene with its native promoter (PXa3) from Minghui 63 to two other susceptible japonica rice varieties, Zhonghua 11 and 02428. A total of 12 independent transformants (MKbZH) in a Zhonghua 11 background were generated. Eight of the nine positive transgenic plants were highly resistant to Xoo strain PXO61 at adult (booting) stage, with lesion areas ranging from 0.4 ± 0.2% to 3.3 ± 2.0%, as compared to 39.0 ± 11.9% and 25.6 ± 4.5% measured for the controls of susceptible Zhonghua 11 and moderately resistant Minghui 63, respectively (Figure 1A, supplemental Table 2 at http://www.genetics.org/supplemental/). The bacterial growth analysis demonstrated that the growth rate of PXO61 on resistant transgenic plants at the booting stage was 87-fold lower than that on wild type (Figure 1B). T1 families derived from three of the resistant T0 plants carrying one copy of Xa3 were further examined individually for resistance by inoculating with PXO61 and also for the presence of the transgene by PCR analysis at booting stage. It was shown that the resistance cosegregated with Xa3 in all three T1 families (supplemental Table 2), indicating that the improved resistance was due to the existence of Xa3. Resistant T1 plants from the MKbZH2 family were further examined for their resistance spectra. Transgenic plants showed significantly enhanced (P < 0.01) resistance to five (PXO61, PXO86, PXO79, PXO71, and PXO145) of the seven strains representing different Xoo races compared with wild type and Minghui 63 at booting stage (Table 1). The transgenic plants were also more resistant than transgenic line Rb17-2 carrying one copy of PXa3:Xa3 in the background of japonica Mudanjiang 8 (Table 1).
Figure 1.—
Performance of Xa3 in different rice lines. Zhonghua 11, Mudanjiang 8, 02428, and Minghui 63 are wild types. Minghui 63 is also the donor of Xa3. The Xa3 gene was driven by native promoter PXa3 in plants named with prefix MKbZH, MKb024, or Rb; by PUbi in plants named MKbFZH, MKbFMDJ, or MKbFMH; and by PWRKY13 in plants 12IMKbZH and 12IMKbMDJ. (A) Leaves from transgenic plants and wild types of booting stage at 14 days after inoculation with Xoo strain PXO61. Rb17-2 was a homozygote transgenic line. MKbFMDJ2 and 12IMKbMDJ7 were resistant T1 plants, and other transgenic plants were T0 generation. N, negative transgenic plants. (B) Growth of PXO61 in leaves of T1 transgenic plants at booting stage. The bacterial population was determined from three leaves at each time point by counting colony-forming units (Sun et al. 2004). (C) Growth of PXO61 in leaves of T1 transgenic plants at the four-leaf stage.
TABLE 1.
Resistance spectrum (lesion area in percentage) of transgenic plants in japonica Zhonghua 11 background at booting stage
| Transgenic line
|
Zhonghua 11 (wild type) | Xa3 donor Minghui 63 | Transgenic line Rb17-2a | ||
|---|---|---|---|---|---|
| Xoo strain | MKbZH2a | MKbFZH2a | |||
| PXO61 | 2.1 ± 0.6* | 0.9 ± 0.3* | 31.2 ± 4.1 | 14.4 ± 0.9 | 3.3 ± 1.7 |
| PXO86 | 0.4 ± 0.1* | 0.3 ± 0.0* | 34.9 ± 4.3 | 9.1 ± 1.5 | 2.4 ± 1.2 |
| PXO79 | 0.7 ± 0.3* | 0.4 ± 0.1* | 33.5 ± 8.5 | 10.5 ± 3.4 | 2.8 ± 1.0 |
| PXO71 | 0.9 ± 0.3* | 0.4 ± 0.2* | 33.6 ± 10.9 | 57.8 ± 9.9 | 3.0 ± 1.1 |
| PXO99 | 14.9 ± 1.9 | 10.9 ± 1.3* | 16.2 ± 2.1 | 68.3 ± 4.9 | 83.8 ± 6.8 |
| PXO145 | 0.6 ± 0.1* | 0.3 ± 0.1* | 24.3 ± 6.3 | 22.9 ± 3.9 | 2.6 ± 1.5 |
| Z173 | 28.1 ± 4.3 | 26.5 ± 2.2* | 35.6 ± 6.2 | 63.0 ± 7.4 | 81.7 ± 4.4 |
Significant difference (P < 0.01) was detected compared with wild type.
MKbZH2, resistant T1 plants carrying one copy of PXa3:Xa3; MKFZH2, resistant T1 plants carrying one copy of PUbi:Xa3. Rb17-2 carries one copy of PXa3:Xa3 with the background of japonica Mudanjiang 8.
Two independent positive transformants (MKb024) in the background of japonica variety 02428 were obtained. The T0 transgenic plants were highly resistant to Xoo strain PXO61, with lesion areas of 3.1 ± 0.3% and 3.2 ± 0.2%, as compared to 47.5 ± 9.4% and 23.9 ± 2.2% measured for the controls of susceptible 02428 and moderately resistant Minghui 63 at booting stage, respectively (Figure 1A, supplemental Table 2 at http://www.genetics.org/supplemental/). The resistance of the T1 family cosegregated with Xa3 (supplemental Table 2), indicating that the improved resistance was due to Xa3. These results suggest that genetic background has a large influence on the function of Xa3 and that a japonica background facilitates Xa3 function more than an indica background.
Host background-enhanced resistance is associated with increased expression of Xa3:
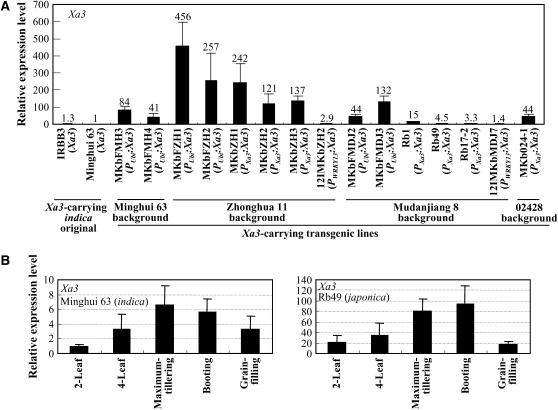
To determine whether genetic background influenced the expression of Xa3, we quantified its transcripts in different rice lines by RT–qPCR. In addition to Minghui 63, the indica line IRBB3 also carries the Xa3 gene (Sun et al. 2004; Xiang et al. 2006). Transgenic lines Rb1, Rb49, and Rb17-2, carrying one copy of Xa3 driven by a native promoter in the genetic background of japonica Mudanjiang 8, showed enhanced resistance compared with the native rice lines carrying Xa3 (Sun et al. 2004). Compared with Minghui 63 and IRBB3, transgenic lines carrying one copy of Xa3 driven by a native promoter in the genetic backgrounds of Zhonghua 11 and Mudanjiang 8 showed 3- to 242-fold more Xa3 transcripts (Figure 2A). Transgenic plants carrying PXa3:Xa3 in the 02428 background had 44-fold more Xa3 transcripts. The data also showed that the expression level of Xa3 was remarkably higher in the Zhonghua 11 background than in Mudanjiang 8 and 02428 backgrounds (Figure 2A). These results suggest that increased Xa3 transcripts may be associated with the enhanced resistance in the transgenic plants with a japonica background.
Figure 2.—
Expression level of Xa3 in different rice lines. Each RNA sample was from the mixture of at least three plants. (A) Genetic background influenced Xa3 expression. Transgenic plants named with prefix MKbFMH, MKbFZH, MKbZH, 12IMKbZH, MKbFMDJ, 12IMKbMDJ, and MKb024-1 were resistant T1 plants, and plants named with prefix Rb were homozygote transgenic lines. All the transgenic plants, except MKbFMH and MKb024-1, in which copy numbers were not determined, carried one copy of Xa3. Xa3 was driven by the native promoter (PXa3), maize ubiquitin gene promoter (PUbi), or pathogen-induced OsWRKY13 gene promoter (PWRKY13) in the transgenic plants. (B) Developmental stage influenced Xa3 expression. The expression level of Xa3 in each developmental stage of each rice line was relative to that in the two-leaf stage of Minghui 63.
To evaluate the above hypothesis, we transferred Xa3 driven by a strong constitutive promoter, maize ubiquitin gene promoter (PUbi), into Zhonghua 11, Mudanjiang 8, and Minghui 63. Eight of the 10 positive T0 plants, all 10 positive T0 plants, and 11 of the 12 positive T0 plants transformed with PUbi:Xa3 in backgrounds of Zhonghua 11, Mudanjiang 8, and Minghui 63, respectively, were highly resistant to PXO61 at booting stage (Figure 1A, supplemental Table 2 at http://www.genetics.org/supplemental/). The lesion area of these highly resistant plants ranged from 0.2 ± 0.1% to 1.5 ± 0.5% in the Zhonghua 11 background as compared to 39.0 ± 11.9% and 25.6 ± 4.5% measured for the susceptible wild-type and moderately resistant Minghui 63 controls, respectively, and from 2.0 ± 0.4% to 8.4 ± 0.6% in the Minghui 63 background as compared to 53.2 ± 2.8% measured for the moderately resistant wild type. Similar results were also obtained for the transgenic plants carrying PUbi:Xa3 in the Mudanjiang 8 background (supplemental Table 2). T1 families derived from 2–3 of the resistant T0 plants from each genetic background were further examined. It was shown that the resistance cosegregated with Xa3 in all the T1 families examined (supplemental Table 2), indicating that the enhanced resistance was due to the transgene Xa3.
Resistant T1 plants MKbFZH2 carrying PUbi:Xa3 in the Zhonghua 11 background were further examined for their resistance spectra. Transgenic plants showed significantly enhanced (P < 0.01) resistance to all seven Xoo strains compared with wild type (Table 1). The MKbFZH2 plants also appeared to be more resistant to Xoo strains PXO61, PXO79, PXO71, PXO99, PXO145, and Z173 than transgenic plants MKbZH2 carrying PXa3:Xa3, as determined by a comparison of lesion areas at booting stage (Table 1). A bacterial growth analysis also indicated that plants carrying PUbi:Xa3 were more resistant to Xoo infection than plants carrying PXa3:Xa3 in the Zhonghua 11 background; the bacterial growth rate of PXO61 on MKbFZH plants was 1.5-fold lower than that on MKbZH plants at 14 days after inoculation (Figure 1B). Similar results were also obtained in transgenic plants in the Mudanjiang 8 background. Resistant T1 plants MKbFMDJ4, MKbFMDJ5, and MKbFMDJ7 carrying PUbi:Xa3 appeared to be more resistant to strains PXO61, PXO79, PXO71, PXO145, PXO280, T7174, T7147, T7133, and Z173 than transgenic line Rb17-2 carrying PXa3:Xa3, as determined by a comparison of lesion areas at booting stage (Table 2). The bacterial growth rate of PXO61 on MKbFMDJ plants was also 1.5-fold lower than that on Rb17-2 plants at 14 days after inoculation (Figure 1B). Overexpression of Xa3 enhanced rice resistance not only in plants in the japonica background but also in those in the indica background. Transgenic plants MKbFMH2, MKbFMH3, MKbFMH4, MKbFMH6, and MKbFMH7 carrying PUbi:Xa3 in the Minghui 63 background showed significantly enhanced (P < 0.01) resistance to six (PXO61, PXO79, PXO71, PXO145, T7133, and Z173) of the seven Xoo strains as compared to the donor of Xa3, Minghui 63, at booting stage (Table 2).
TABLE 2.
Resistance spectrum (lesion area in percentage) of transgenic plants in japonica Mudanjiang 8 and indica Minghui 63 backgrounds at booting stage
| Mudanjiang 8 backgrounda
|
Minghui 63 backgrounda
|
||||
|---|---|---|---|---|---|
| Xoo strain | Rb17-2b | MKbFMDJ4, -5, -7b | Mudanjiang 8 (wild type) | MKbFMH2, -3, -4, -6, -7b | Minghui 63 (wild type) |
| PXO61 | 2.4 ± 0.8** | 1.5 ± 0.7** | 88.7 ± 10.2 | 8.0 ± 5.7** | 46.1 ± 9.4 |
| PXO79 | 1.5 ± 0.3** | 0.8 ± 0.1** | 89.6 ± 19.0 | 3.4 ± 1.5** | 38.7 ± 8.2 |
| PXO71 | 3.6 ± 1.8** | 0.7** | 81.9 ± 13.7 | 7.9 ± 4.9** | 42.7 ± 9.6 |
| PXO99 | 48.0 ± 16.9 | 54.3 ± 9.9 | 46.1 ± 6.2 | 59.0 ± 24.8 | 62.4 ± 14.0 |
| PXO145 | 4.3 ± 0.9** | 0.7 ± 0.1** | 39.8 ± 8.8 | 9.3 ± 2.8** | 35.4 ± 10.8 |
| PXO280 | 12.0 ± 7.6** | 1.2 ± 0.1** | 59.5 ± 16.8 | ||
| T7174 | 18.5 ± 6.8 | 5.9 ± 0.6* | 29.0 ± 14.7 | ||
| T7147 | 24.9 ± 10.2 | 3.5 ± 1.8** | 21.4 ± 6.3 | ||
| T7133 | 2.2 ± 1.0** | 1.1 ± 0.5** | 85.7 ± 12.9 | 4.0 ± 1.0** | 44.4 ± 19.1 |
| Z173 | 25.4 ± 4.5 | 8.1 ± 6.6 | 29.1 ± 3.2 | 36.4 ± 6.2** | 52.5 ± 6.8 |
Significant difference (P < 0.05) was detected compared with wild type. **Significant difference (P < 0.01) was detected compared with wild type.
Plants with the two genetic backgrounds were inoculated with Xoo strains at different times.
Rb17-2, homozygote transgenic line carrying one copy of PXa3:Xa3; MKbFMDJ4, -5, -7, resistant T1 plants carrying one copy of PUbi:Xa3; MKbFMH2, -3, -4, -6, -7, resistant T0 plants carrying PUbi:Xa3.
We also transferred Xa3 driven by a weak and pathogen-induced promoter, rice OsWRKY13 gene promoter (PWRKY13) (Qiu et al. 2007), into Zhonghua 11 and Mudanjiang 8. All 16 and 4 positive T0 plants in Zhonghua 11 and Mudanjiang 8 backgrounds, respectively, carrying PWRKY13:Xa3 showed enhanced resistance as compared to wild type; this enhanced resistance cosegregated with Xa3 in T1 families (Figure 1A, supplemental Table 2 at http://www.genetics.org/supplemental/). However, Xa3-mediated resistance was significantly impaired (P < 0.01) in plants carrying PWRKY13:Xa3 as compared to plants carrying PXa3:Xa3 in the same genetic background. The average lesion area of transgenic plants carrying PWRKY13:Xa3 was ∼3- to 11-fold larger than that of the transgenic plants carrying PXa3:Xa3 in the same genetic background on infection (Table 3). The bacterial growth rate on plants carrying PWRKY13:Xa3 was 7.9- and 29.5-fold higher than that on plants carrying PXa3:Xa3 in Zhonghua 11 and Mudanjiang 8 backgrounds, respectively (Figure 1B).
TABLE 3.
Comparison of PXa3:Xa3- and PWRKY13:Xa3-mediated resistance (lesion area in percentage) to Xoo strain PXO61 at booting stage
| Zhonghua 11 background
|
Mudanjiang 8 background
|
|||
|---|---|---|---|---|
| MKbZH (PXa3) | 12IMKbZH (PWRKY13) | Rb17-2 (PXa3) | 12IMKbMDJ (PWRKY13) | |
| T0 plant | ||||
| Range | 0.4 – 3.3 | 7.7 – 25.7 | 36.2 – 53.1 | |
| Average | 1.3 ± 1.0 | 14.2 ± 5.5* | 41.5 ± 7.9 | |
| T1 plant | ||||
| Range | 0.8 – 6.8 | 3.9 – 16.2 | 1.7 – 7.3 | 19.1 – 42.0 |
| Average | 2.5 ± 1.7 | 7.5 ± 3.2* | 5.4 ± 0.9 | 22.9 ± 7.4* |
All positive transgenic plants are shown in supplemental Table 2. Rb17-2 is the homozygote transgenic line. *Significant difference (P < 0.01) from plants carrying PXa3:Xa3 was detected.
The Xa3 expression level driven by PUbi was, on average, 2-, 11-, and 63-fold higher than that driven by the native promoter in the backgrounds of Zhonghua 11, Mudanjiang 8, and Minghui 63, respectively. The Xa3 expression level driven by PWRKY13 was only, on average, 2 and 18% of that driven by the native promoter in the backgrounds of Zhonghua 11 and Mudanjiang 8, respectively (Figure 2A). The negative correlation between lesion area and Xa3 expression level in the plants shown in Figure 2A was −0.523, significant at α = 0.05 (n = 17). The variable resistance ability of plants carrying Xa3 driven by different promoters and different expression levels of Xa3 suggests that the function of Xa3 is associated with its expression level: The higher its expression, the more resistant the plant.
Developmentally controlled Xa3 activity is associated with its expression level:
Minghui 63 and IRBB3 were susceptible to Xoo strains PXO61 and PXO71 at seedling stage (Table 4). However, Minghui 63 became moderately resistant or moderately susceptible to PXO61, although still susceptible to PXO71, and IRBB3 became resistant to PXO61 and PXO71 at adult (booting) stage (Tables 1 and 2) (Sun et al. 2004). Plants carrying PXa3:Xa3 in the background of japonica variety Mudanjiang 8 were highly resistant to these Xoo strains at seedling stage (Table 4) (Sun et al. 2004). Transgenic plants carrying PXa3:Xa3 in Zhonghua 11 and 02428 backgrounds were also highly resistant to Xoo strains at seedling (four-leaf) stage as compared to Minghui 63 and IRBB3 (Table 4). The growth rates of PXO61 on resistant transgenic plants carrying PXa3:Xa3 in Zhonghua 11, Mudanjiang 8, and 02428 backgrounds were 28-, 16-, and 13-fold lower than those on Minghui 63 at 12 days after bacterial infection at the four-leaf stage, respectively (supplemental Figure 2 at http://www.genetics.org/supplemental/, Figure 1C). The bacterial growth rates on transgenic plants carrying PUbi:Xa3 in Zhonghua 11, Mudanjiang 8, and Minghui 63 backgrounds were 25-, 44-, and 101- to 207-fold lower than the growth rate on Minghui 63 at the four-leaf stage, respectively.
TABLE 4.
Reaction (lesion area in percentage) of transgenic plants carrying PXa3:Xa3 in different japonica backgrounds at seedling (four-leaf) stage
|
Indica variety (Xa3)
|
Zhonghua 11 background
|
02428 background
|
Mudanjiang 8 background
|
|||||
|---|---|---|---|---|---|---|---|---|
| Xoo strain | Minghui 63 | IRBB3 | Transgenica | Zhonghua 11 (wild type) | Transgenicb | 02428 (wild type) | Transgenicc | Mudanjiang 8 (wild type) |
| PXO61 | 70.7 ± 4.4 | 75.4 ± 3.8 | 5.2 ± 1.3 | 59.2 ± 6.3 | 9.3 ± 1.1 | 100.0 ± 0.0 | 6.0 ± 1.5 | 100.0 ± 0.0 |
| PXO71 | 76.1 ± 8.7 | 75.3 ± 6.7 | 6.6 ± 2.3 | 51.0 ± 7.4 | 10.3 ± 2.0 | 100.0 ± 0.0 | 5.4 ± 2.6 | 100.0 ± 0.0 |
Resistant T1 plants MKbZH2.
Resistant T1 plants MKb024-1.
Resistant transgenic line Rb17-2.
To examine whether Xa3 is expressed differentially at different developmental stages, Minghui 63 and transgenic line Rb49 carrying PXa3:Xa3 in the background of japonica variety Mudanjiang 8 were grown with staggered planting so that RNA samples were obtained from plants at the two-leaf, four-leaf, maximum-tillering, booting, and grain-filling stages at the same time from different varieties. RT–qPCR analysis showed that the Xa3 expression level was very low at the two-leaf stage, gradually increased with development, and reached the highest level at the maximum-tillering or booting stage in both Minghui 63 and Rb49 (Figure 2B). However, Xa3 transcript levels in Rb49 were ∼21-, 11-, 12-, 17-, and 5-fold higher than those in Minghui 63 from the two-leaf to the grain-filling stages, respectively. The association between increasing Xa3-mediated resistance and Xa3 expression level accompanying development suggests that the developmentally controlled disease resistance in the indica background plants is Xa3-dosage dependent.
Increasing Xa3 expression results in enhanced expression of defense-responsive genes:
The expression of rice OsWRKY13, encoding a transcription factor, was rapidly induced in incompatible (resistant) host–pathogen interaction and lightly induced in compatible (susceptible) host–pathogen interaction; overexpression of OsWRKY13 enhanced rice resistance to Xoo (Wen et al. 2003; Qiu et al. 2007). NH1 is the rice ortholog of Arabidopsis NPR1; this gene was rapidly induced in incompatible host–pathogen interaction as compared to compatible interaction and overexpression of NH1 enhanced rice resistance to Xoo (Chern et al. 2005; Yuan et al. 2007). These results suggest that OsWRKY13 and NH1 are involved in R gene-mediated resistance against Xoo. To determine the role of OsWRKY13 and NH1 in genetic background-influenced and Xa3-mediated resistance, we analyzed their expression in rice lines with different expression levels of Xa3. RT–qPCR analysis showed that plants with more Xa3 transcripts (Figure 2A) induced the expression of OsWRKY13 and NH1 more rapidly and/or effectively upon bacterial infection (Figure 3). Japonica transgenic lines Rb49 and MKbZH1 carrying one copy of PXa3:Xa3 had 5.6- and 15-fold more OsWRKY13 transcripts and 2.1- and 1.6-fold more NH1 transcripts than indica line Minghui 63 carrying Xa3 as compared with the maximum transcript level within 1 day postinfection. The same two transgenic lines had 1.2- and 3.1-fold more OsWRKY13 transcripts and 3.9- and 2.9-fold more NH1 transcripts than indica line IRBB3 carrying Xa3. Transgenic line MKbFZH2 carrying the strong expression construct PUbi:Xa3 had 2-fold more OsWRKY13 transcripts than MKbZH1 in the same genetic background, although the maximum transcript level of NH1 in MKbFZH2 was slightly lower than that in MKbZH1. However, the maximum transcript levels of OsWRKY13 and NH1 in transgenic line 12IMKbZH2 carrying the weak expression construct PWRKY13:Xa3 were only 59 and 36%, respectively, of those in MKbZH1. Consistent with the expression pattern reported previously (Qiu et al. 2007; Yuan et al. 2007), both resistant and susceptible responses in the same genetic background induced OsWRKY13 and NH1, but the former reaction resulted in more OsWRKY13 and NH1 transcripts than the latter as compared with the maximum transcript level within 1 day postinfection (Figure 3).
Figure 3.—
Expression levels of OsWRKY13 and NH1 in different rice lines. Plants were inoculated with Xoo strain PXO61 at booting stage. ck, without inoculation. The expression level of the genes in each time point of each rice line was relative to that in the ck of Minghui 63.
Pathogen infection differentially influences Xa3 expression in plants with different genetic backgrounds:
Xa3 expression was suppressed (∼2.5- to 3-fold) at 4 hr postinoculation and then induced (∼2- to 4-fold) as compared to noninfected plants in indica rice lines Minghui 63 and IRBB3 (Figure 4). This suppression was not observed in transgenic lines carrying PXa3:Xa3 in japonica backgrounds. In contrast, pathogen infection induced (∼5.5- to 7-fold) Xa3 expression in transgenic lines Rb49 and Rb17-2 in the genetic background of japonica variety Mudanjiang 8 (Figure 4). The expression level of Xa3 in transgenic lines MKbZH1 and MKbZH2 in the background of japonica Zhonghua 11 showed no remarkable differences before and after pathogen infection. Pathogen infection showed ∼10-fold induction of Xa3 in the transgenic plants 12IMKbZH2 carrying one copy of PWRKY13:Xa3 with a Zhonghua 11 background at 12 hr after infection, although the induced transcript level was still ∼1.5-fold lower than that in noninoculated MKbZH2 plants carrying PXa3:Xa3 (Figure 4). These results suggest that genetic background also influences Xa3 expression in response to bacterial invasion.
Figure 4.—
Xa3 expression on pathogen infection analyzed by RT–qPCR. Plants were inoculated with Xoo strain PXO61 at booting stage. ck, without inoculation; ckM, transgenic plant MKbZH2 without inoculation.
DISCUSSION
The present results confirm our previous finding that a japonica background facilitates the function of Xa3 more than an indica background (Sun et al. 2004). These results are also consistent with the identification and application of Xa3 in rice production. This gene was first identified in japonica variety Wase Aikoku 3 (Ezuka et al. 1975) and is an important resistance gene in japonica cultivar breeding in China (Xu et al. 2004), one of the largest rice-growing countries in the world.
The resistance spectrum conferred by an R gene is related to pathogen recognition specificity. Although the amino acid sequence, especially the LRR sequence, of LRR-containing R proteins is the major determinant of pathogen recognition (Dangl and Jones 2001), our results indicate that the expression level of an R gene can also influence the resistance spectrum conferred by this gene. Increasing Xa3 expression can enlarge the resistance spectrum mediated by Xa3. Studies of other genes also support that some R gene-mediated resistance has a dosage effect. Overexpression of tomato R gene Pto, encoding a serine/threonine protein kinase, activates defense responses and confers broad resistance (Tang et al. 1999). Overexpression of tomato Prf and Arabidopsis RPS2, encoding nucleotide-binding site (NBS)–LRR-type proteins, leads to constitutive activation of the defense response and broad-spectrum resistance, respectively (Oldroyd and Staskawicz 1998; Tao et al. 2000). Overexpression of the tomato LRR membrane protein type R gene in Nicotiana benthamiana also induces a constitutive defense response (Wulff et al. 2004). However, not all R genes can mediate an enlarged resistance spectrum by overexpression. Overexpression of the Arabidopsis SSI4 gene, encoding Toll interleukin 1 receptor–NBS–LRR protein, failed to enhance disease resistance, while its mutant allele, ssi4, encoding a protein with a single-amino-acid substitution in the NBS domain, showed enhanced resistance to bacterial and oomycete pathogens (Shirano et al. 2002).
The putative mechanisms that enhanced resistance associated with increased expression of Xa3 include the following: First, increasing XA3 proteins may facilitate their interaction with different pathogen effectors or guardees, the pathogenicity targets of the host (Dangl and Jones 2001). The interaction specificity between an R protein and pathogen effector or the host guardee should determine the pathogen recognition efficiency of a host. Highly specific host–pathogen interaction may require only small amounts of R proteins, which may explain why R genes usually show a low level of expression (De Ilarduya and Kaloshian 2001; Shen et al. 2002; Paal et al. 2004; Schornack et al. 2004; Sun et al. 2004; Huang et al. 2005). Otherwise, large amounts of R proteins are needed for nonperfect interaction. It has been reported that overexpression of a pathogen effector avrBs3 causes a loss of recognition specificity of tomato R protein Bs4 (Schornack et al. 2004). The enlarged resistance spectrum mediated by Xa3 in the japonica background and in overexpression status may be due to the loss of perception specificity of the XA3 protein to some bacterial effectors. However, plants carrying PUbi:Xa3 could not confer full resistance to Xoo strains PXO99 and Z173, indicating that overexpression of Xa3 caused only a partial, but not complete, loss of recognition specificity among different Xoo races. Second, increasing XA3 proteins may facilitate more rapid or effective initiation of defense signaling transduction during host–pathogen interaction, which resulted in reduced lesion area and bacterial growth rate. Both OsWRKY13 and NH1 are involved in R gene-mediated Xoo resistance and they are dosage dependent in bacterial resistance; OsWRKY13 and NH1 are transcript regulators that directly or indirectly control the expression of a subset of defense-responsive genes (Wen et al. 2003; Chern et al. 2005; Qiu et al. 2007; Yuan et al. 2007). Rice lines with more Xa3 transcripts induced OsWRKY13 and NH1 more rapidly and/or effectively as compared with the rice lines with less Xa3 transcripts or without carrying Xa3. These results suggest that rapid activation of OsWRKY13- and NH1-involved defense signal transduction might partly explain the enhanced resistance associated with increased expression of Xa3.
Developmentally controlled resistance has been observed in many plant–pathogen systems. Full disease resistance usually occurs at adult stages in these systems. Rice Xa21 is expressed at both susceptible and resistant stages, indicating that Xa21-mediated developmentally controlled disease resistance may not be related to its expression (Century et al. 1999). Xa3 and Xa21 encoding the same type of proteins share 53% sequence similarity (Sun et al. 2004). However, the present results indicate that the developmentally controlled Xa3-mediated resistance to some Xoo strains is associated with its expression level. This dosage-dependent developmental control may also be related to bacterial recognition specificity. Minghui 63 was highly resistant to Xoo strain JL691 at both seedling and adult stages (Chen et al. 2002), suggesting that XA3 can efficiently and specifically perceive the effector of JL691 and that more XA3 proteins are required for recognition of PXO61. R genes often express constitutively in either uninfected or infected plants (De Ilarduya and Kaloshian 2001; Shen et al. 2002; Paal et al. 2004; Huang et al. 2005; Schornack et al. 2005), which agrees with their common role in pathogen recognition. This indicates that in most cases, the basal level of R proteins preexisting in cells is sufficient to guard pathogen invasion and initiate host resistance. However, in a few cases, pathogen induction increases R gene expression (Thurau et al. 2003; Levy et al. 2004; Gu et al. 2005). These results suggest that more R proteins are required on infection to help amplify the resistance response. Xa3 belongs to the latter group of R genes. Low levels of pathogen-induced Xa3 expression were constantly observed in indica rice lines and japonica transgenic lines in the Mudanjiang 8 background. This result is consistent with the observation that increasing Xa3 expression can enhance rice resistance. This induction was not detected in japonica transgenic lines in the Zhonghua 11 background, which may be due to very high levels of Xa3 transcripts in these plants masking the light induction. However, a suppression of Xa3 expression was also observed only in indica rice lines at early infection (4 hr). Further study is needed to determine whether this is one of the causes of the impaired disease resistance in indica lines as compared to japonica transgenic plants.
Xa3 preferentially expresses in the cells surrounding the vascular vessels (Y. Cao and S. Wang, unpublished data), which perfectly fits the function of genes conferring resistance to Xoo, a vascular pathogen. Functional overlap between pathogen-induced defense signaling and plant development has been reported (Holt et al. 2002; Godiard et al. 2003; Chu et al. 2006), which may partly explain the fitness cost in disease resistance. Constitutive expression of an R gene sometimes results in plants with abnormal morphology or decreased fertility. Overexpression of the tomato Pto gene caused constitutive cell death (Tang et al. 1999). Arabidopsis overexpressing RPW8 was lethal (Xiao et al. 2003). Even the native expression of RPM1 influenced Arabidopsis development (Tian et al. 2003). Interestingly, Xa3-overexpressing plants showed no remarkable morphologic and developmental differences from wild type, which may contribute to, at least partly, the restricted expressional location of Xa3. Thus overexpression of Xa3 can be applied to breeding programs to produce whole-growth-stage and wide-resistant-spectrum rice.
Acknowledgments
This work was supported by grants from the National Program of High Technology Development of China and the National Natural Science Foundation of China.
References
- Banerjee, D., X. Zhang and A. F. Bent, 2001. The leucine-rich repeat domain can determine effective interaction between RPS2 and other host factors in Arabidopsis RPS2-mediated disease resistance. Genetics 158: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir, Y., R. Subramaniam and J. L. Dangl, 2004. Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr. Opin. Plant. Biol. 7: 391–399. [DOI] [PubMed] [Google Scholar]
- Century, K. S., R. A. Lagman, M. Adkisson, J. Morlan, R. Tobias et al., 1999. Developmental control of Xa21-mediated disease resistance in rice. Plant J. 20: 231–236. [DOI] [PubMed] [Google Scholar]
- Chen, H., S. Wang and Q. Zhang, 2002. A new gene for bacterial blight resistance in rice located on chromosome 12 identified from Minghui 63, an elite restorer line. Phytopathology 92: 750–754. [DOI] [PubMed] [Google Scholar]
- Chern, M. S., H. A. Fitzgerald, P. E. Canlas, D. A. Navarre and P. C. Ronald, 2005. Overexpression of a rice NPR1 homolog leads to constitutive activation of defense response and hypersensitivity to light. Mol. Plant-Microbe Interact. 18: 511–520. [DOI] [PubMed] [Google Scholar]
- Chu, Z., M. Yuan, J. Yao, X. Ge, B. Yuan et al., 2006. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 20: 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J. L., and J. D. Jones, 2001. Plant pathogens and integrated defence responses to infection. Nature 411: 826–833. [DOI] [PubMed] [Google Scholar]
- De Ilarduya, O. M., and I. Kaloshian, 2001. Mi-1.2 transcripts accumulate ubiquitously in resistant Lycopersicon esculentum. J. Nematol. 33: 116–120. [PMC free article] [PubMed] [Google Scholar]
- Ellis, J. G., G. J. Lawrence, J. E. Luck and P. N. Dodds, 1999. The identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezuka, A., O. Horino, K. Toriyama, H. Shinoda and T. Morinaka, 1975. Inheritance of resistance of rice variety Wase Aikoku 3 to Xanthomonas oryzae. Bull. Tokai-Kinki Natl. Agric. Exp. Stn. 28: 124–130. [Google Scholar]
- Godiard, L., L. Sauviac, K. U. Torii, O. Grenon, B. Mangin et al., 2003. ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. Plant J. 36: 353–365. [DOI] [PubMed] [Google Scholar]
- Goel, R. K., and A. K. Gupta, 1990. Host age in relation to resistance in rice to bacterial blight caused by Xanthomonas campestris pv. oryzae. Trop. Agric. 67: 368–370. [Google Scholar]
- Gu, K., B. Yang, D. Tian, L. Wu, D. Wang et al., 2005. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 435: 1122–1125. [DOI] [PubMed] [Google Scholar]
- Holt, B. F., D. C. Boyes, M. Ellerstrom, N. Siefers, A. Wiig et al., 2002. An evolutionarily conserved mediator of plant disease resistance gene function is required for normal Arabidopsis development. Dev. Cell 2: 807–817. [DOI] [PubMed] [Google Scholar]
- Huang, S., E. A. van der Vossen, H. Kuang, V. G. Vleeshouwers, N. Zhang et al., 2005. Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J. 42: 251–261. [DOI] [PubMed] [Google Scholar]
- Levy, M., O. Edelbaum and H. Sela, 2004. Tobacco mosaic virus regulates the expression of its own resistance gene N. Plant Physiol. 135: 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and Q. Zhang, 2005. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 23: 540–547. [DOI] [PubMed] [Google Scholar]
- Luck, J. E., G. J. Lawrence, P. N. Dodds, K. W. Shepherd and J. G. Ellis, 2000. Regions outside of the Leucine-rich repeats of flax rust resistance proteins play a role in specificity determination. Plant Cell 12: 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, G. B., A. J. Bogdanove and G. Sessa, 2003. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54: 23–61. [DOI] [PubMed] [Google Scholar]
- Mew, T. W., 1987. Current status and future prospects of research on bacterial blight of rice. Annu. Rev. Phytopathol. 25: 359–382. [Google Scholar]
- Ogawa, T., 1993. Methods, and strategy for monitoring race distribution and identification of resistance to bacterial leaf blight (Xanthomonas campestris pv. oryzae) in rice. Jpn. Agric. Res. Q. 27: 71–80. [Google Scholar]
- Ogawa, T., T. Yamamoto, G. S. Khush and T. W. Mew, 1991. Breeding of near-isogenic lines of rice with single genes for resistance to bacterial blight pathogen (Xanthomonas campestris pv. oryzae). Jpn. J. Breed. 41: 523–529. [Google Scholar]
- Oldroyd, G. E., and B. J. Staskawicz, 1998. Genetically engineered broad-spectrum disease resistance in tomato. Proc. Natl. Acad. Sci. USA 95: 10300–10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paal, J., H. Henselewski, J. Muth, K. Meksem, C. M. Menendez et al., 2004. Molecular cloning of the potato Gro1–4 gene conferring resistance to pathotype Rol of the root cyst nematode Globodera rostochiensis. Plant J. 38: 285–297. [DOI] [PubMed] [Google Scholar]
- Panter, S. N., K. E. Hammond-Kosack, K. Harrison, J. D. Jones and D. A. Jones, 2002. Developmental control of promoter activity is not responsible for mature onset of Cf-9B-mediated resistance to leaf mold in tomato. Mol. Plant-Microbe Interact. 15: 1099–1107. [DOI] [PubMed] [Google Scholar]
- Qiu, D., J. Xiao, X. Ding, M. Xiong, M. Cai et al., 2007. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant-Microbe Interact. 20: 492–499. [DOI] [PubMed] [Google Scholar]
- Schornack, S., A. Ballvora, D. Gurlebeck, J. Peart, D. Baulcombe et al., 2004. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 37: 46–60. [DOI] [PubMed] [Google Scholar]
- Schornack, S., K. Peter, U. Bonas and T. Lahaye, 2005. Expression levels of avrBs3-like genes affect recognition specificity in tomato Bs4- but not in pepper Bs3-mediated perception. Mol. Plant-Microbe Interact. 18: 1215–1225. [DOI] [PubMed] [Google Scholar]
- Shen, K. A., D. B. Chin, R. Arroyo-Garcia, O. E. Ochoa, D. O. Lavelle et al., 2002. Dm3 is one member of a large constitutively expressed family of nucleotide binding site-leucine-rich repeat encoding genes. Mol. Plant-Microbe Interact. 15: 251–261. [DOI] [PubMed] [Google Scholar]
- Shirano, Y., P. Kachroo, J. Shah and D. F. Klessig, 2002. A gain-of-function mutation in an Arabidopsis Toll interleukin 1 receptor-nucleotide binding site-leucine-rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell 14: 3149–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Y. Cao, Z. Yang, C. Xu, X. Lie et al., 2004. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 37: 517–527. [DOI] [PubMed] [Google Scholar]
- Tang, X., M. Xie, Y. J. Kim, J. Zhou, D. F. Klessig et al., 1999. Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell 11: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., F. Yuan, R. T. Leister, F. M. Ausubel and F. Katagiri, 2000. Mutational analysis of the Arabidopsis nucleotide binding site-leucine-rich repeat resistance gene RPS2. Plant Cell 12: 2541–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurau, T., S. Kifle, C. Jung and D. Cai, 2003. The promoter of the nematode resistance gene Hs1pro-1 activates a nematode-responsive and feeding site-specific gene expression in sugar beet (Beta vulgaris L.) and Arabidopsis thaliana. Plant Mol. Biol. 25: 643–660. [DOI] [PubMed] [Google Scholar]
- Tian, D., M. B. Traw, J. Q. Chen, M. Kreitman and J. Bergelson, 2003. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423: 74–77. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn, R. A., R. Roth and P. J. de Wit, 2001. Identification of distinct specificity determinants in resistance protein Cf-4 allows construction of a Cf-9 mutant that confers recognition of avirulence protein Avr4. Plant Cell 13: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, N., Z. Chu and S. Wang, 2003. Three types of defense-responsive genes are involved in resistance to bacterial blight and fungal blast diseases in rice. Mol. Genet. Genomics 269: 331–339. [DOI] [PubMed] [Google Scholar]
- Wulff, B. B., M. Kruijt, P. L. Collins, C. M. Thomas, A. A. Ludwig et al., 2004. Gene shuffling-generated and natural variant of the tomato resistance gene Cf-9 exhibit different auto-necrosis-inducing activities in Nicotiana species. Plant J. 40: 942–956. [DOI] [PubMed] [Google Scholar]
- Xiang, Y., Y. Cao, C. Xu, X. Li and S. Wang, 2006. Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor. Appl. Genet. 113: 1347–1355. [DOI] [PubMed] [Google Scholar]
- Xiao, S., S. Brown, E. Patrick, C. Brearley and J. G. Turner, 2003. Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid-dependent amplification circuit is required for hypersensitive cell death. Plant Cell 15: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Q. Sun, F. Liu, Z. Chen, B. Hu et al., 2004. Race Monitoring of rice bacterial blight (Xanthomonas oryzae pv. oryzae) in China. Chin. J. Rice Sci. 18: 469–472. [Google Scholar]
- Yuan, Y., S. Zhong, Q. Li, Z. Zhu, Y. Lou et al., 2007. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotech. J. 5: 313–324. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., and T. W. Mew, 1985. Adult-plant resistance of rice cultivars to bacterial blight. Plant Dis. 69: 896–898. [Google Scholar]