Abstract
In vivo, microdamage occurs in the form of linear microcracks and diffuse damage. However, it is unknown whether the age-related changes in bone quality predispose bone to form one type of damage morphology over the other during in vivo loading. In this study, histological and histomorphometrical analyses were conducted on transverse cross sections, obtained from the tibiae of aging human bone (age 19 to 89), to investigate the in vivo accumulation and localization of damage morphologies. The results demonstrate that old donor bone (83 ± 3 years) contains more linear microcracks than younger donor bone in the cortices predominantly subjected to compressive (p < 0.01) and tensile loading (p < 0.01). In contrast, young donor bone (40 ± 10 years) contains more diffuse damage than older donor bone in the cortex predominantly subjected to tensile loading (p < 0.01). The formation of damage morphology showed no correlation with bone geometry parameters and exhibited distinct preferences with bone microstructure. Linear microcracks formed in the interstitial bone (p < 0.01) and were either trapped or arrested by the microstructural interfaces (cement line and lamellar interface) (p < 0.05). Areas of diffuse damage, however, were preferentially associated with secondary osteonal bone (p < 0.01) and had no relationship with the microstructural interfaces (p < 0.01). Based upon these findings, we conclude that age-related changes in bone microstructure, but not bone geometry, play a key role in the propensity of old donors to form linear microcrack over diffuse damage under in vivo loading conditions.
Keywords: Aging, Microcracks, Diffuse Damage, In vivo Loading, Bone Quality
INTRODUCTION
It is well known that both cancellous and cortical tissue contribute to the fracture resistance of bone [1,2]. Quantitative or qualitative changes in cancellous bone and in cortical bone are therefore expected to affect bone fragility. Historically, only the quantitative changes, measured by bone mass, have been considered to be a significant predictor of fracture risk [3]. However, recent evidence shows that not all the variability in bone fracture can be explained by bone mass alone [4–9]. For example, Kanis [2005] noted that even though the changes in BMD (bone mineral density) between the ages of fifty and eighty years predict a four-fold increase in hip fracture risk, the risk in reality increased thirty-fold. Thus, age–related changes in bone quality could affect the mechanical competence of bone including the formation of microdamage.
In vivo microdamage occurs in the form of linear microcracks [10–13] and diffuse damage [14,15]. Previous studies provide evidence that different damage morphologies (linear microcracks and diffuse damage) have different effects on the mechanical properties of bone [16–19]. For example, Burr et al. [1998] demonstrated that stiffness loss has a linear relationship with diffuse damage and a quadratic relationship with linear microcracks. They also found that tensile damage accumulates more easily but crack growth is greater in compression [16]. We showed that cortical bone compartmentalizes the damage morphologies in different regions and the sequence of damage production in different phases of cyclic loading to dissipate energy and resist a catastrophic fracture [18]. More significantly, the propensity of older human bone to form linear microcracks over diffuse damage has been suggested to play a critical role in bone fragility [19].
Using an in vitro model, damage morphology has shown to be a combined function of local strain and tissue properties [20, 16, 17, 18, 19]. Linear microcracks form under compressive loading [20, 16, 17, 18, 19] in the interstitial bone and either stop at the cement lines (i.e the osteonal boundaries) [20] or develop along the lamellar interfaces (i.e. the interlamellar boundaries) [19]. In contrast, diffuse damage forms under tensile loading [20, 17, 18] as submicroscopic cracks [20] and has no relationship with the lamellar arrangement of bone [19]. In addition to the tissue properties, bone geometry could also affect damage morphology. Specifically, narrower tibias have been shown to fail in a brittle manner [21] suggesting that bone width may play a role in the development of damage morphology. The interaction of strain mode (tensile vs. compressive) with bone microstructure and geometry may therefore alter the mode and magnitude of damage formation with aging under in vivo loading conditions.
Thus, the aim of this study was to determine the in vivo occurrence and accumulation of linear microcracks and diffuse damage in human cortical bone and to investigate their relationship with bone microstructure and geometry.
MATERIALS AND METHODS
Transverse sections were obtained from twenty-four human cadaveric tibiae (Age range 19 to 89 years; Sex: males and females) who died suddenly showing no apparent bone disease. These sections were separated into two groups (Young, mean age = 40 ± 10 years, N = 11, Nmale = 6, Nfemale = 5; and Old, mean age = 83 ± 3 years, N = 13, Nmale = 7, Nfemale = 6). The preparation of sections included cutting a 1 cm long segment from the distal diaphysis followed by en bloc staining in 1% basic fuchsin [22]. After staining, the segments were embedded in polymethyl methacrylate (PMMA) and serial transverse sections (100 μm thickness) were obtained. The center of each section was located 3.5 cm below the middle of the diaphysis. This technique of staining and sectioning is known to mark only the damage present in bone at the time of the donor’s death [23]. Transverse sections, and not longitudinal sections, were used because the distinction between the interstitial and secondary osteonal bone is often difficult in longitudinal sections [24, 19].
Histological analyses of microdamage
Microdamage was measured in the anterior and posterior cortices at 200× magnification using bright-field microscopy (IX81, Olympus, Melville, NY 11747). The anterior and posterior cortices were chosen because under in vivo loading they are subjected primarily to tensile and compressive loading, respectively [Peterman et al. 2001] [25]. In basic fuchsin stained sections, linear microcracks appear as a sharply defined line (Figure 1) and diffuse damage appears as an area of pooled staining (Figure 1) [20, 15, 18, 19]. Selective areas containing diffuse damage or a linear microcrack were examined under a laser confocal microscope (Zeiss, Thornwood, NY 10594; Excitation = 543 nm; Emission = 560 nm) to verify the presence of damage (Figure 1). Following the histological assessment of microdamage, the bone sections were subjected to geometric and microstructural analyses.
Figure 1.
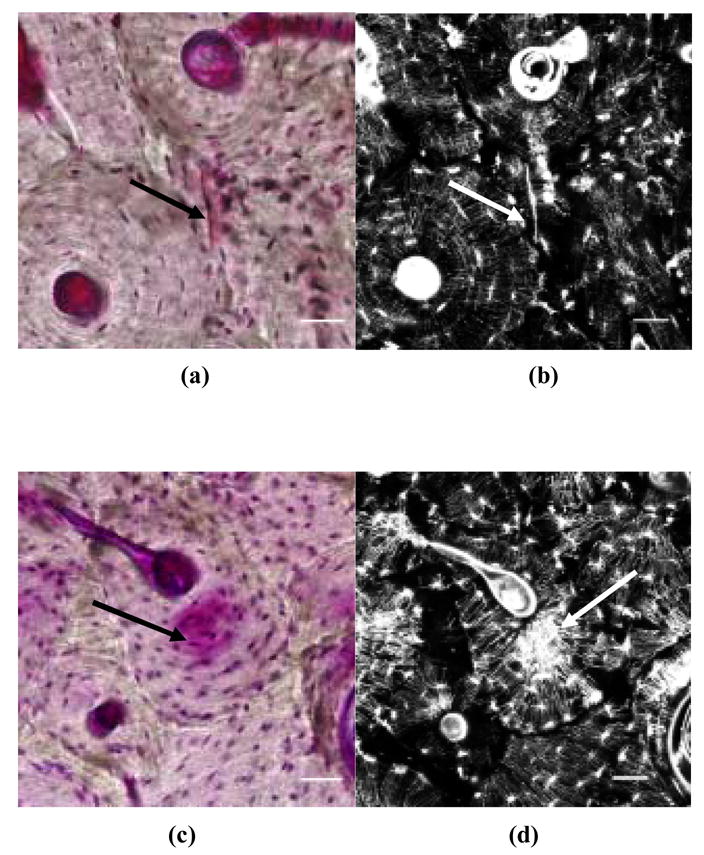
In vivo linear microcracks (a: under bright-field microscopy; b: under a laser confocal microscope) and diffuse damage (c: under bright-field microscopy; d: under a laser confocal microscope) in human cortical bone. Scale bars = 50 μm.
Geometric analyses
The transverse sections were scanned and converted to 8 bit BMP digital images using Adobe Photoshop 5.5 (Adobe Systems Inc., San Jose, CA, 95110). In conjunction with Image J 1.36 [http://rsb.info.nih.gov/ij/], a macro (computer program), available for the estimation of cross sectional moments [http://www.hopkinsmedicine.org/FAE/mmacro.htm], was used to calculate the principal moments of inertia (Imax, Imin), polar moment of inertia (J), anterior cortex width (Aw), posterior cortex width (Pw), tibia width in the anterior-posterior direction (APw), section modulus in the anterior-posterior direction (Sm.AP), polar moment of inertia normalized to tibia length (J/L), and cortical area (Ct. Ar) of the bone cross sections [21, 26]. The macro provides a better estimate of the principal moments of inertia than the two concentric ellipse method [27, 28]. To avoid any operator bias in the calculation of cortex width, the anterior cortex width (Aw) and the posterior cortex width (Pw) were measured where the axis, representing the maximum bending rigidity, crossed the anterior and posterior cortices, respectively.
Microstructural analyses
Because of donor-to-donor variability in areas of interstitial and osteonal bone, linear microcracks and diffuse damage were normalized to interstitial/osteonal areas. The interstitial and secondary osteonal bone areas were determined by taking images from the anterior and posterior cortices under brightfield microscopy at 125 × magnification. Average measurements were taken over four fields to reduce any measurement errors [29, 30]. The quantities measured from each selected field included the total area of the field, total area of secondary osteons including haversian canals, total area of the haversian canals, and total area of all pores (> 200 μm2) including the haversian canals [31, 30, 26]. The osteon fragments that had a distinct haversian canal and a clear cement line were included in the quantification of the secondary osteonal area [26]. The microstructural parameters of interest were calculated as follows [31, 30, 26]:
| (1) |
| (2) |
| (3) |
To analyze the role of the microstructural interfaces in the development of damage morphology, linear microcracks and diffuse damage were counted based on the following classification (Figure 2): LM1: linear microcrack that either formed at the lamellar interface or was stopped at the cement line; LM2: linear microcrack that neither formed at the lamellar interface nor stopped at the cement line; DD1: diffuse damage patch that was either limited to one lamella or stopped at the cement line; and DD2: diffuse damage patch that was neither limited to one lamella nor stopped at the cement line. The lamellar interface, defined as the boundary between adjacent lamellae, was visualized using a bright-field microscopy with a polarizing filter [19, 32].
Figure 2.
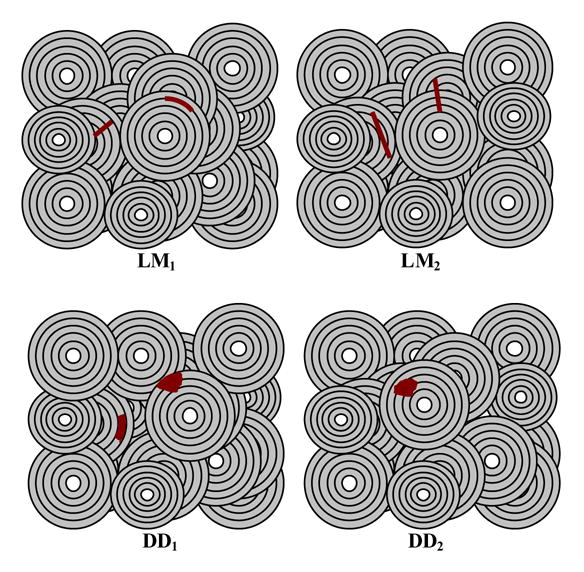
Schematic representation of microdamage morphologies and their relationship with the microstructural interfaces. LM1: linear microcrack that either formed at the lamellar interface or was stopped at the cement line. LM2: linear microcrack that neither formed at the lamellar interface nor was stopped at the cement line. DD1: diffuse damage patch that was either limited to one lamella or was stopped at the cement line. DD2: diffuse damage patch that was neither limited to one lamella nor was stopped at the cement line.
Statistical analyses
Following a check for normality, the differences in microdamage accumulation [linear microcrack density (#/mm2), diffuse damage density (mm2/mm2)] first between males and females and then between the young and old groups were compared by an unpaired t-test. For those variables failing the normality test, a Mann-Whitney test was used for comparison. The association between damage morphology and bone microstructure was determined by comparing the number of linear microcracks associated with interstitial bone area (#/mm2) vs. osteonal bone area (#/mm2) and the diffuse damage areas associated with interstitial bone area (mm2/mm2) vs. osteonal bone area (mm2/mm2). The effects of the microstructural interfaces on damage morphology were determined by comparing the number of LM1 vs. number of LM2 and the number of DD1 areas vs. the number of DD2 areas. Either a paired t-test or a Wilcoxon signed-rank test was used for all the above comparisons. Linear regression analysis was conducted to examine the relationship between the geometrical parameters and damage morphology. SigmaStat 2.0 (SYSTAT Software Inc, Chicago, IL 60606) was used for all the statistical analyses.
RESULTS
Because no gender-related differences in morphology or accumulation of microdamage were found (p > 0. 05) for both the young and old groups, data from males and female donors were pooled for microdamage comparisons. Damage accumulation and morphology were significantly different between the young and old groups. Compared to young donors, cortical bone cross sections obtained from old donors contained 3.5-fold more linear microcracks in the cortex subjected to compressive loading (posterior cortex) (p < 0.01, Figure 3a) and nearly 3-fold more linear microcracks in the cortex subjected to tensile loading (anterior cortex) (p < 0.01, Figure 3a). In contrast, bones obtained from young donors contained 25-fold more diffuse damage than bones obtained from old donors in the cortex subjected to tensile loading (anterior cortex) (p < 0.01, Figure 3b). Diffuse damage accumulation in the cortex subjected to compressive loading (posterior cortex) was minor and not statistically significant between the young and old groups (p = 0.662, Figure 3b).
Figure 3.
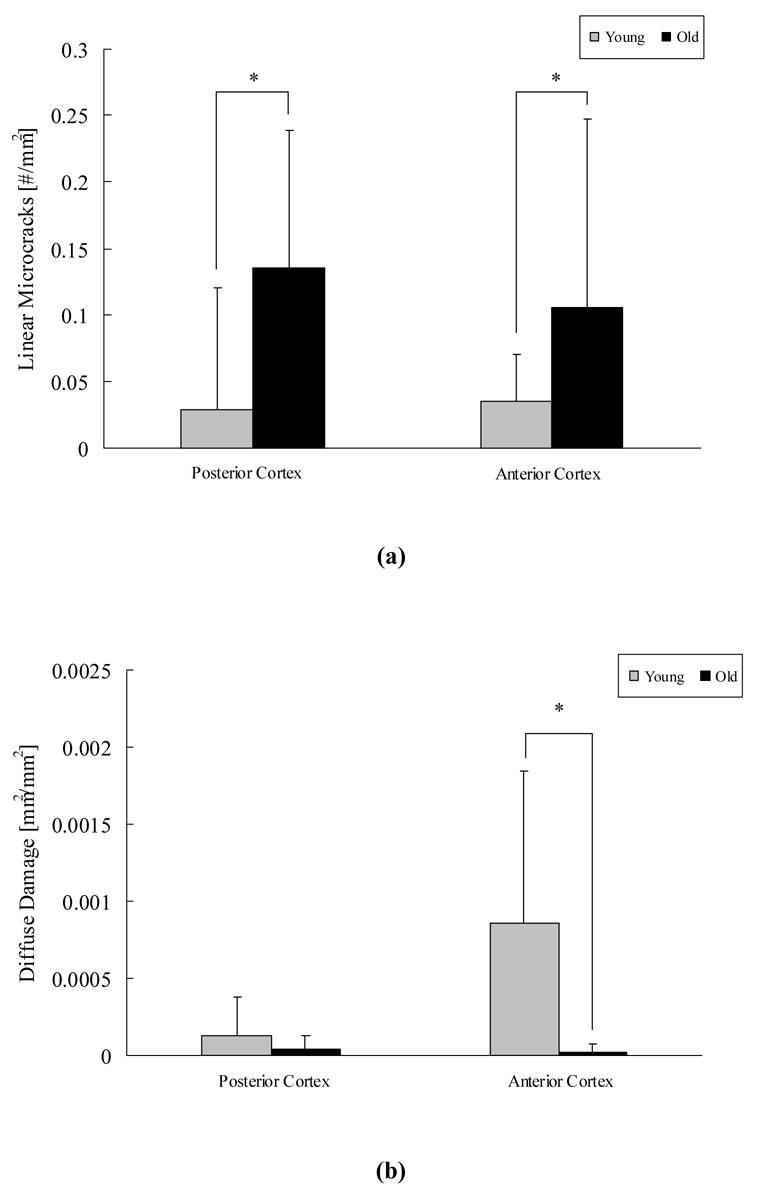
Bones obtained from old donors contained more linear microcracks than the bones from young donors in both the anterior and posterior cortices (a). In contrast, bones obtained young donors contained more diffuse damage than bones from old donors in the anterior cortex (b). * indicates p < 0.05.
The differences in the development of microdamage between the young and old donors could not be fully explained by bone geometry. Both morphologies of damage had weak correlations with bone geometrical parameters (Table 1). Damage morphology, however, exhibited different preferences with bone microstructure. Twenty-two-fold more linear microcracks formed within interstitial bone than secondary osteonal bone (p < 0.01, Figure 4a) while nearly 4-fold more diffuse damage developed in secondary osteonal bone than interstitial bone for both young and old donors (p < 0.01, Figure 4b).
Table 1.
Correlation between damage morphology and the geometric parameters. The first and second rows correspond to the r2 and p values, respectively. Significant correlations are shown in bold.
| Imax | Imin | J | Aw | Pw | APw | Sm. AP | J/L | Ct.Ar | |
|---|---|---|---|---|---|---|---|---|---|
| LM Posterior Cortex | 0.011
0.633 |
0.007
0.690 |
0.010
0.645 |
N/A | 0.003
0.801 |
0.003
0.787 |
0.015
0.565 |
0.017
0.547 |
0.001
0.907 |
| LM Anterior Cortex | 0.003
0.802 |
0.001
0.904 |
0.001
0.898 |
0.011
0.631 |
N/A | 0.030
0.419 |
0.000
0.998 |
0.003
0.787 |
0.005
0.737 |
| DD Posterior Cortex | 0.026
0.453 |
0.001
0.858 |
0.015
0.570 |
N/A |
0.271
0.009 |
0.039
0.358 |
0.008
0.676 |
0.007
0.691 |
0.068
0.220 |
| DD Anterior Cortex | 0.084
0.171 |
0.038
0.361 |
0.069
0.214 |
0.089
0.157 |
N/A | 0.057
0.261 |
0.040
0.351 |
0.035
0.380 |
0.095
0.143 |
Figure 4.
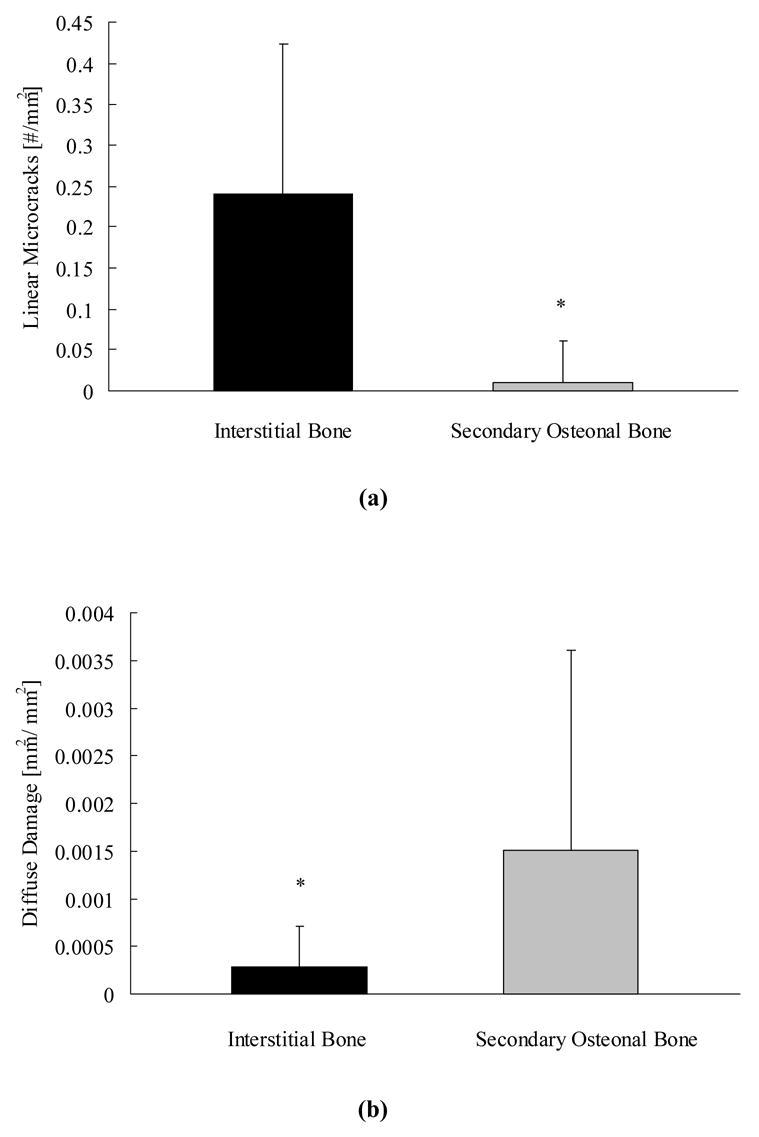
More linear microcracks formed in interstitial bone than secondary osteonal bone (a), while more diffuse damage developed in secondary osteonal bone than interstitial bone (b). * indicates p < 0.05.
Linear microcracks and diffuse damage showed distinct relationships with the microstructural interfaces for both young and old donors. A post-hoc analysis revealed that 88% of the linear microcracks either developed along the lamellar interface or were stopped at the cement line (p < 0.05, Figures 2 and 5a). In contrast, 79% of the diffuse damage patches were neither limited to one lamella nor were stopped at the cement line (p < 0.01, Figures 2 and 5b).
Figure 5.
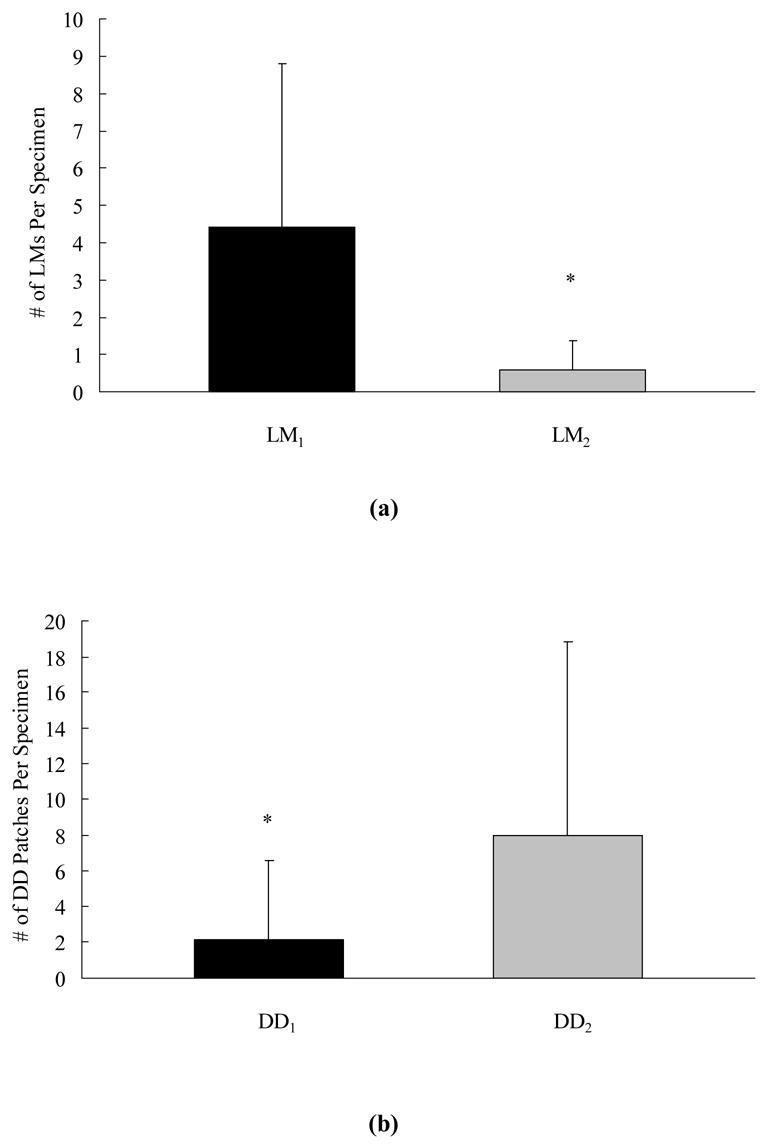
More linear microcracks either formed at the lamellar interface or were stopped at the cement line (LM1) (a). In contrast, diffuse damage patches were neither limited to one lamella nor were stopped at the cement line (DD2) (b). * indicates p < 0.05.
DISCUSSION
The differences in linear microcrack formation between the young and old donors found in this investigation are consistent with previous studies that showed an age-related increase in linear microcracks density [11–13]. Characterization of age-related changes in in vivo damage morphology has not been reported previously. To our knowledge this is the first study to report the effects of age on damage morphology development and to identify the relationship between damage morphology and the microstructural interfaces in vivo.
Here, we investigated the incidence and accumulation of linear microcracks and diffuse damage in the anterior and posterior cortices of the tibia. The anterior and posterior cortices were chosen because dynamic gait simulation of human cadaveric tibia shows that the primary loading on the tibia is bending and that the maximum tensile and compressive strains occur in the anterior and posterior cortices, respectively [25]. Unlike experiments done on human subjects, where strain gages were placed either on the antero-medial or on the medial aspect of the tibia [33, 34, 35], the human cadaveric model allows strains to be recorded from all the four cortices and therefore provides a more comprehensive information about the tibia loading milieu [25].
In a previous investigation, we reported that diffuse damage forms at an early stage in the fatigue life while the formation of linear microcracks occurs towards the end of the fatigue life [18]. The ability of cortical bone to compartmentalize damage morphology production in different stages of cyclic loading, therefore, plays an important role in resisting a catastrophic fracture [18]. Results of this study show that, under in vivo loading, bones obtained from young donors have a higher propensity than bones obtained from old donors to form diffuse damage that is known to dissipates more energy and is self-limiting (does not grow further) [36, 16, 18]. More importantly here we show that in addition to forming significantly less diffuse damage in the tensile cortex, the aging human bone forms significantly more linear microcracks than young human bone in both the tensile and compressive cortices. Linear microcracks are traditionally associated with compressive loading and the terminal phase of bone fracture characterized by rapid crack propagation and catastrophic fracture [16, 18]. These results suggest that other modes of loading including mixed mode loading may combine with changes associated with advancing age including the decline in bone tissue quality and consequently dominate the formation of linear microcracks in both the tensile and compressive cortices [37].
In vivo microdamage accumulation depends on the imbalance between the rate at which the remodeling process repairs damage and the rate at which microdamage is initiated [38]. The accumulation of in vivo damage reported in this study could therefore be a result of several factors including bone quality, applied load, or/and a decline in the remodeling rate. In a previous study [19], we have used an in vitro model to examine the tissue level mechanical properties (bone quality) and found that specimens obtained from old donors form more linear microcracks than specimens obtained from young donors while young donors form more diffuse damage than old donors under similar loading conditions [19]. Thus, bone quality plays a role in damage morphology development. However, a decline in the remodeling rate will exacerbate the effects of bone quality and result in the accumulation of more microdamage.
A recent study by Tommasini et al. [2005] have demonstrated that in young males bone fragility can also be predicted by bone geometry. Bones obtained from narrower tibias were more brittle and accumulated more damage compared to bones obtained from wider tibias [21]. Here, we show that only the formation of diffuse damage in the posterior cortex has a significant correlation with cortex width (r2 = 0.271). However, the magnitude of diffuse damage accumulation in the posterior cortex was very limited and showed no difference between the young and old groups (Figure 3b). Therefore, bone geometry may be a good predictor for stress fracture risk in young adults, but it does not predict the age-related changes in in vivo damage morphology where other factors including bone turnover-mediated changes in bone quality may play a role in microdamage development.
Specifically in contrast to bone geometry, the changes in the turnover-based bone quality parameters including the interstitial and secondary osteonal content of bone can provide a better understanding of factors that predispose bone to form one type of damage morphology over the other, as shown by the localization of linear microcracks and diffuse damage with interstitial and secondary osteonal bone, respectively (Figures 4a and 4b). Interstitial and secondary osteonal bone are reported to have different chemical composition [39, 40] and mechanical properties [41, 39]. Interstitial bone is characterized as an older tissue that is more highly mineralized [39, 40] and stiffer [41, 39] than secondary osteonal bone. Consequently, interstitial bone is more likely to dissipate energy in a brittle manner [42] by forming linear microcracks [43, 44] over diffuse damage, as was found here.
The relationship between different damage morphologies and microstructural interfaces was also distinct. Eighty-eight percent of the linear microcracks either followed the lamellar interface or stopped at the cement line (Figures 2 and 5a). However, only 21% of the diffuse damage patches were related to the microstructural interfaces (Figures 2 and 5b). Thus, the lamellar interface and the cement line in bone are associated with the arrest and trapping of linear microcracks but not diffuse damage.
Previous studies show that the lamellar interface [40, 19] and the cement line [45, 11, 12, 20, 46, 47] affect bone toughness differently. Jepsen et al. [1999] and Diab et al. [2006] demonstrated that the lamellar interface has an intimate relationship with linear microcracks suggesting that the orientation of the lamellae may play a role in resisting crack propagation [19]. The preferential orientation of the lamellae parallel to the longitudinal axis of bone [48] could provide bone with toughness [49, 50]. Additionally the lamellar interface also keeps the cracks physically isolated from each other which in turn delays microcrack coalescence [40]. On the other hand, the cement line is known to contribute to bone toughness by acting as a barrier to crack propagation or by deflecting crack growth [45, 11, 12, 20, 46, 47]. Moshin et al. [2006] found that microcracks less than 100 micron in length stopped at the cement line while microcracks in the range of 100–300 micron were deflected as they encountered the osteonal boundaries. Therefore, despite the reduced propensity of older donor bone to form diffuse damage, the microstructural interfaces can still act to provide the bone with some toughening mechanisms to resist linear microcrack propagation (Figure 5a).
The disassociation between diffuse damage and the microstructural interfaces (Figure 5b) also suggest that age-related changes at the ultrastructural level may have a greater affect on the formation of diffuse damage than linear microcracks. However, the age-related variations investigated in this study are limited to the effects of bone microstructure on damage morphology development, and no attempt has been made to identify the ultrastructural features associated with these changes. For example, it is known that aging bone is characterized by an increase in the fraction of highly mineralized bone [51] and the modification of collagen by denaturation [52] or non-enzymatic glycation [53, 54]. All the above factors may make bone more prone to form linear microcracks over diffuse damage and should be investigated further.
A limitation of this work is that linear microcracks and diffuse damage were observed only in the transverse sections. In a previous study [19] we examined longitudinal sections and found that cortical bone displays comparable trends in damage morphology development to what was reported here. Hence, it is reasonable to consider that the orientation of the sections will not affect the morphology and accumulation of microdamage although the actual length of microcracks may be longer in the longitudinal sections [55].
In conclusion, the results of this investigation demonstrate that age-related changes associated with bone quality (as measured by bone microstructure) affect the propensity of bone to form one mode of damage morphology over the other. Following in vivo loading, old donor bone contained more linear microcracks than young donors in the cortex subjected to compressive loading and the cortex subjected to tensile loading. In contrast, young donors formed more diffuse damage than old donors in the cortex subjected to tensile loading. The formation of damage morphology had distinct relationships with bone microstructure. Linear microcracks and diffuse damage colocalized with interstitial and secondary osteonal bone, respectively. Furthermore, linear microcracks were more often arrested by the microstructural (lamellar and cement line) interfaces than was diffuse damage. A better understanding of the relationship between bone quality and damage morphology can provide a better insight into the age-related increase in bone fragility.
Acknowledgments
This study is supported by NIH grant AR 49635. Thanks to the National Disease Research Interchange (Washington DC) for providing the human tissue. Thanks to Dr. David B. Burr for providing critical input to improve this manuscript and Dr. Keith W. Condon for preparation of histological sections.
Footnotes
Submitted to BONE
Ms. Ref. No.: BONE-D-06-00378
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burr DB, Turner CH. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. J Bone Miner Res. 2005:58–65. [Google Scholar]
- 2.Eswaran SK, Gupta A, Adams MF, Keaveny TM. Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res. 2006;21:307–314. doi: 10.1359/jbmr.2006.21.2.307. [DOI] [PubMed] [Google Scholar]
- 3.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 4.Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol. 1987;30:789–811. doi: 10.1097/00003081-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCabe F, Zhou LJ, Steele CR, Marcus R. Noninvasive assessment of ulnar bending stiffness in women. J Bone Miner Res. 1991;6:53–59. doi: 10.1002/jbmr.5650060110. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 8.Jepsen KJ, Schaffler MB. ORS Transactions. Vol. 26. San Francisco: California; 2001. Bone mass does not adequately predict variations in bone fragility: A genetics approach. [Google Scholar]
- 9.Kanis, Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. J Bone Miner Res. 2005:316–323. [Google Scholar]
- 10.Frost HM. Presence of microscopic cracks in vivo in bone. Henry Ford Hosp Med Bull. 1960;8:25–35. [Google Scholar]
- 11.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 12.Norman TL, Wang Z. Microdamage of human cortical bone: incidence and morphology in long bones. Bone. 1997;20:375–9. doi: 10.1016/s8756-3282(97)00004-5. [DOI] [PubMed] [Google Scholar]
- 13.Zioupos P. Accumulation of in-vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc. 2001;201(Pt 2):270–278. [PubMed] [Google Scholar]
- 14.Fazzalari NL, Forwood MR, Smith K, Manthey BA, Herreen P. Assessment of cancellous bone quality in severe osteoarthrosis: bone mineral density, mechanics, and microdamage. Bone. 1998;22:381–388. doi: 10.1016/s8756-3282(97)00298-6. [DOI] [PubMed] [Google Scholar]
- 15.Vashishth D, Koontz J, Qiu SJ, Lundin-Cannon D, Yeni YN, Schaffler MB, Fyhrie DP. In vivo diffuse damage in human vertebral trabecular bone. Bone. 2000;26:147–152. doi: 10.1016/s8756-3282(99)00253-7. [DOI] [PubMed] [Google Scholar]
- 16.Burr DB, Turner CH, Naick P, Forwood MR, Ambrosius W, Hasan MS, Pidaparti R. Does microdamage accumulation affect the mechanical properties of bone? J Biomech. 1998;31:337–345. doi: 10.1016/s0021-9290(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 17.Reilly GC, Currey JD. The effects of damage and microcracking on the impact strength of bone. J Biomech. 2000;33:337–343. doi: 10.1016/s0021-9290(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 18.Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37:96–102. doi: 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Diab T, Condon KW, Burr DB, Vashishth D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone. 2006;38:427–431. doi: 10.1016/j.bone.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. J Orthop Res. 1998;16:322–329. doi: 10.1002/jor.1100160308. [DOI] [PubMed] [Google Scholar]
- 21.Tommasini SM, Nasser P, Schaffler MB, Jepsen KJ. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005;20:1372–80. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]
- 22.Burr DB, Hooser M. Alterations to the en bloc basic fuchsin staining protocol for the demonstration of microdamage produced in vivo. Bone. 1995;4:431–433. doi: 10.1016/s8756-3282(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 23.Burr DB, Stafford T. Validity of the bulk-staining technique to separate artifactual from in vivo bone microdamage. Clin Orthop Relat Res. 1990;260:305–8. [PubMed] [Google Scholar]
- 24.Diab T, Sit S, Kim DG, Rho JY, Vashishth D. Age-dependent fatigue behaviour of human cortical bone. 2005 Feb–Apr;42(1–2):53–59. doi: 10.1080/09243860500095539. [DOI] [PubMed] [Google Scholar]
- 25.Peterman MM, Hamel AJ, Cavanagh PR, Piazza SJ, Sharkey NA. In vitro modeling of human tibial strains during exercise in micro-gravity. J Biomech. 2001;34:693–698. doi: 10.1016/s0021-9290(01)00004-5. [DOI] [PubMed] [Google Scholar]
- 26.Ural A, Vashishth D. Interactions between microstructural and geometrical adaptation in human cortical bone. J Orthop Res. 2006;24:1489–1498. doi: 10.1002/jor.20159. [DOI] [PubMed] [Google Scholar]
- 27.Ruff C. http://www.hopkinsmedicine.org/FAE/mmacro.htm.
- 28.Medalia AI. Dynamic shape factors of particles. Powder Technology. 1970;4:117–138. [Google Scholar]
- 29.Martin RB, Pickett JC, Zinaich S. Studies of skeletal remodeling in aging men. Clin Orthop Relat Res. 1980;149:268–82. [PubMed] [Google Scholar]
- 30.Yeni YN, Brown CU, Wang Z, Norman TL. The influence of bone morphology on fracture toughness of the human femur and tibia. Bone. 1997;21:453–9. doi: 10.1016/s8756-3282(97)00173-7. [DOI] [PubMed] [Google Scholar]
- 31.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 32.Jepsen KJ, Davy DT, Krzypow DJ. The role of the lamellar interface during torsional yielding of human cortical bone. J Biomech. 1999;32:303–310. doi: 10.1016/s0021-9290(98)00179-1. [DOI] [PubMed] [Google Scholar]
- 33.Lanyon LE, Hampson WG, Goodship AE, Shah JS. Bone deformation recorded in vivo from strain gauges attached to the human tibia. Acta Orthopaedica Scandinavica. 1975;46:256–258. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 34.Burr DB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, Hoshaw S, Saiag E, Simkin A. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–410. doi: 10.1016/8756-3282(96)00028-2. [DOI] [PubMed] [Google Scholar]
- 35.Milgrom C, Burr D, Fyhrie D, Forwood M, Finestone A, Nyska M, Giladi M, Liebergall M, Simkin A. The effect of shoe gear on human tibial strains recorded during dynamic loading: a pilot study. Foot Ankle Int. 2006;17:667–71. doi: 10.1177/107110079601701104. [DOI] [PubMed] [Google Scholar]
- 36.Martin RB, Lau ST, Mathews PV, Gibson VA, Stover SM. Collagen fiber organization is related to mechanical properties and remodeling in equine bone. A comparison of two methods. J Biomech. 1996;29:1515–1521. [PubMed] [Google Scholar]
- 37.George WT, Vashishth D. Susceptibility of aging human bone to mixed-mode fracture increases bone fragility. Bone. 2006;38:105–111. doi: 10.1016/j.bone.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Martin RB, Burr DB, Sharkey NA. Skeletal Tissue Mechanics. Springer; 1998. [Google Scholar]
- 39.Rho JY, Zioupos P, Currey JD, Pharr GM. Microstructural elasticity and regional heterogeneity in human femoral bone of various ages examined by nano-indentation. J Biomech. 2002;35:189–198. doi: 10.1016/s0021-9290(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 40.Skedros JG, Holmes JL, Vajda EG, Bloebaum RD. Cement lines of secondary osteons in human bone are not mineral-deficient: new data in a historical perspective. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:781–803. doi: 10.1002/ar.a.20214. [DOI] [PubMed] [Google Scholar]
- 41.Phelps JB, Hubbard GB, Wang X, Agrawal CM. Microstructural heterogeneity and the fracture toughness of bone. J Biomed Mater Res. 2000;51:735–741. doi: 10.1002/1097-4636(20000915)51:4<735::aid-jbm23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 42.Mashiba T, Hui S, Turner CH, Mori S, Johnston CC, Burr DB. Bone remodeling at the iliac crest can predict the changes in remodeling dynamics, microdamage accumulation, and mechanical properties in the lumbar vertebrae of dogs. Calcif Tissue Int. 2005;77:180–185. doi: 10.1007/s00223-005-1295-x. [DOI] [PubMed] [Google Scholar]
- 43.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 44.Wasserman N, Yerramshetty J, Akkus O. Microcracks colocalize within highly mineralized regions of cortical bone tissue. Eur J Morphol. 2005;42(1–2):43–51. doi: 10.1080/09243860500095471. [DOI] [PubMed] [Google Scholar]
- 45.Burr DB, Schaffler MB, Frederickson RG. Composition of the cement line and its possible mechanical role as a local interface in human compact bone. J Biomech. 1988;21:939–945. doi: 10.1016/0021-9290(88)90132-7. [DOI] [PubMed] [Google Scholar]
- 46.Mohsin S, O’Brien FJ, Lee TC. Osteonal crack barriers in ovine compact bone. J Anat. 2006;208:81–89. doi: 10.1111/j.1469-7580.2006.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson VA, Stover SM, Gibeling JC, Hazelwood SJ, Martin RB. Osteonal effects on elastic modulus and fatigue life in equine bone. J Biomech. 2006;39:217–25. doi: 10.1016/j.jbiomech.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Martin RB, Burr DB. Structure, Function, and Adaptation of Compact Bone. Raven Press; 1989. [Google Scholar]
- 49.Vashishth D, Tanner KE, Bonfield W. Contribution, development and morphology of microcracking in cortical bone during crack propagation. J Biomech. 2000;33:1169–1174. doi: 10.1016/s0021-9290(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 50.Taylor D, Tilmans A. Stress intensity variations in bone microcracks during the repair process. J Theor Biol. 2004;229:169–177. doi: 10.1016/j.jtbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Simmons ED, 3rd, Pritzker KP, Grynpas MD. Age-related changes in the human femoral cortex. J Orthop Res. 1991;9:155–167. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Bank RA, TeKoppele JM, Agrawal CM. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19:1021–1026. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 53.Catanese J, Bank RA, TeKoppele JM, Keaveny TM. Increased cross-linking by non-enzymatic glycation reduces the ductility of bone and bone collagen. Proceedings of the ASME 1999 Bioengineering Conference; Bioengineering Division. 1999. pp. 267–268. [Google Scholar]
- 54.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 55.Waserman N, Brydges B, Searles S, Akkus O. Proceedings of the ASME 2006 Bioengineering Conference Bioengineering Division; pp. BIO2006–157515. [Google Scholar]


