Abstract
Partial 16S rDNA from Vibrio collection type strains and recent isolates of Vibrio-related strains were sequenced and compared with previously published sequences. A 24-base DNA oligonucleotide (VaV3) was designed and used as a specific probe for detection and identification of Vibrio anguillarum. Its specificity was tested against collection type strains and environmental isolates and no cross-reaction was found. The probe detected 8 of the 10 V. anguillarum serovars. It was applied to screen different Vibrio-related strains isolated from marine hatcheries and fish farms. The detection limit in DNA-DNA slot blot hybridization was 150 pg.
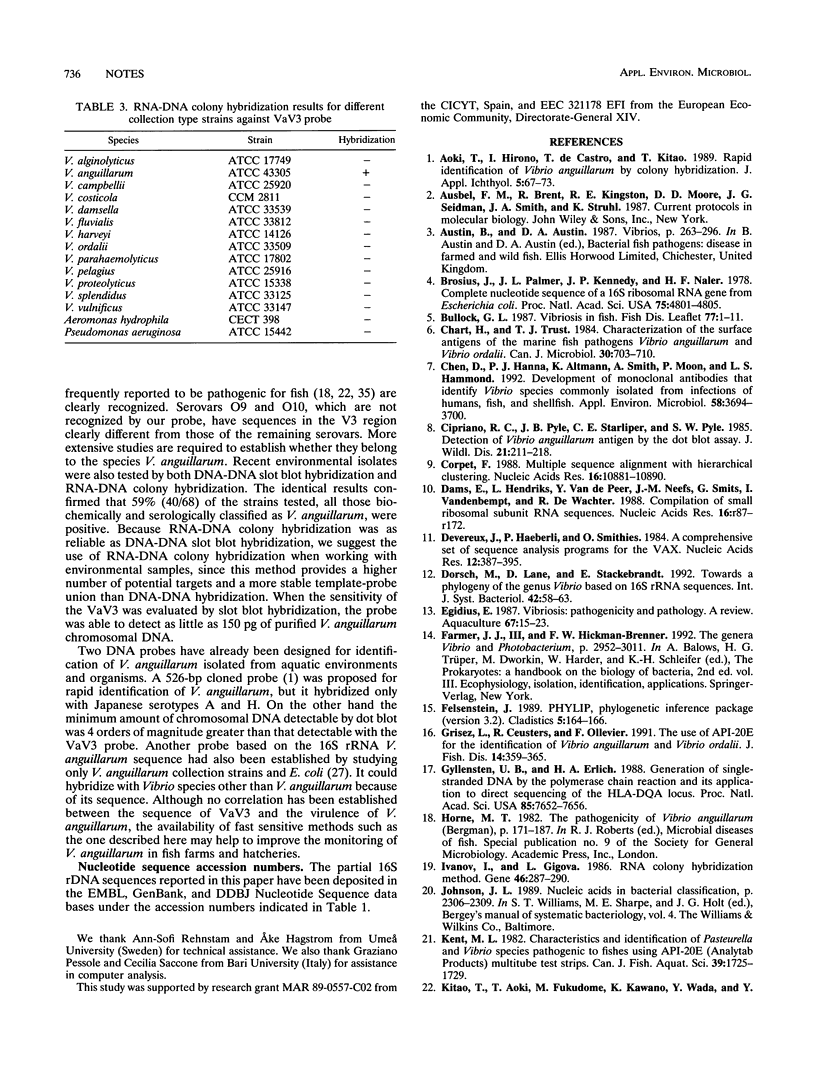
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Trust T. J. Characterization of the surface antigens of the marine fish pathogens Vibrio anguillarum and Vibrio ordalii. Can J Microbiol. 1984 May;30(5):703–710. doi: 10.1139/m84-105. [DOI] [PubMed] [Google Scholar]
- Chen D., Hanna P. J., Altmann K., Smith A., Moon P., Hammond L. S. Development of monoclonal antibodies that identify Vibrio species commonly isolated from infections of humans, fish, and shellfish. Appl Environ Microbiol. 1992 Nov;58(11):3694–3700. doi: 10.1128/aem.58.11.3694-3700.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriano R. C., Pyle J. B., Starliper C. E., Pyle S. W. Detection of Vibrio anguillarum antigen by the dot blot assay. J Wildl Dis. 1985 Jul;21(3):211–218. doi: 10.7589/0090-3558-21.3.211. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch M., Lane D., Stackebrandt E. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int J Syst Bacteriol. 1992 Jan;42(1):58–63. doi: 10.1099/00207713-42-1-58. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I., Gigova L. RNA colony hybridization method. Gene. 1986;46(2-3):287–290. doi: 10.1016/0378-1119(86)90413-0. [DOI] [PubMed] [Google Scholar]
- Kita-Tsukamoto K., Oyaizu H., Nanba K., Simidu U. Phylogenetic relationships of marine bacteria, mainly members of the family Vibrionaceae, determined on the basis of 16S rRNA sequences. Int J Syst Bacteriol. 1993 Jan;43(1):8–19. doi: 10.1099/00207713-43-1-8. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J. G., Gersdorf H., Jansson E., Hongslo T., Göbel U. B., Johansson K. E. Rapid identification of Renibacterium salmoninarum using an oligonucleotide probe complementary to 16S rRNA. Mol Cell Probes. 1993 Feb;7(1):25–33. doi: 10.1006/mcpr.1993.1004. [DOI] [PubMed] [Google Scholar]
- Rehnstam A. S., Norqvist A., Wolf-Watz H., Hagström A. Identification of Vibrio anguillarum in fish by using partial 16S rRNA sequences and a specific 16S rRNA oligonucleotide probe. Appl Environ Microbiol. 1989 Aug;55(8):1907–1910. doi: 10.1128/aem.55.8.1907-1910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone C., Lanave C., Pesole G., Preparata G. Influence of base composition on quantitative estimates of gene evolution. Methods Enzymol. 1990;183:570–583. doi: 10.1016/0076-6879(90)83037-a. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Larsen J. L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986 Mar;51(3):593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]