Abstract
Background and purpose:
Preliminary results in human mesangial cells (MC) suggested that all-trans retinoic acid (ATRA) increased the expression of COX-2 and the production of prostaglandin E2 (PGE2), a PG with anti-inflammatory effects in MC. The aim of this work is to confirm that ATRA increases the expression of COX-2 in MC and to examine the mechanisms involved.
Experimental approach:
Cultured MC were treated with ATRA. COX expression and kinase activity were analyzed by Western blot. Transcriptional mechanisms were analyzed by Northern blot, RT-PCR and promoter assays.
Key results:
COX-2 and COX-1 expression and PGE2 production were increased by ATRA. COX-2 played a role in PGE2 production as production was only partially inhibited by COX-1 inhibitor SC-560. COX-2 up-regulation by ATRA was due to transcriptional mechanisms as pre-incubation with actinomycin D abolished it and ATRA increased the expression of COX-2 mRNA and the activity of a human COX-2 promoter construct, whereas post-transcriptional mechanisms were not found. Retinoic acid receptors (RAR) were not involved in the up-regulation of COX-2 by ATRA since it was not inhibited by RAR-pan-antagonists and the RAR-pan-agonist TTNPB did not up-regulate COX-2. Instead ATRA might act through a sustained activation of extracellular signal-regulated kinase 1/2 (ERK1/2) since up-regulation of COX-2 was prevented by inhibition of the activation of ERK1/2 with PD098059. Also ERK1/2, as well as downstream signalling proteins from ERK1/2, remained phosphorylated when COX-2 increased 24 h later.
Conclusions and implications:
These results highlight the relevance of RAR-independent mechanisms to the biological effects of ATRA.
Keywords: all-trans retinoic acid, cyclooxygenase, mesangial cell, prostaglandin, extracellular signal-regulated kinase 1/2, mitogen- and stress-activated protein kinase-1, cyclic AMP-response-element binding protein
Introduction
Cyclooxygenase (COX), also known as prostaglandin H (PGH) synthase, is a membrane-bound, bifunctional enzyme that catalyses the conversion of arachidonic acid to prostaglandin (PG) PGG2 by its cyclooxygenase activity, and of PGG2 to PGH2 by its peroxidase activity. It is the rate-limiting step in the biosynthesis of biologically active and physiologically important PGs. Up to now, we only know of two COX isoforms that are called COX-1 and COX-2. The COX-1 isoenzyme is constitutively expressed in many tissues and is assumed to be responsible for the physiological functions of PGs such as maintenance of the integrity of gastric mucosa. In contrast, COX-2 is an immediate-early response gene that is undetectable in most mammalian tissues but is rapidly induced by growth factors, tumour promoters, bacterial endotoxins, hypoxia and proinflammatory cytokines such as interleukin-1β (IL-1β) (Smith et al., 2000).
Biologically active retinoids, a family of vitamin A metabolites or analogues, are characterized by their capacity to bind and activate retinoid nuclear receptors, including retinoic acid receptors (RARs) and/or retinoid X receptors (RXRs), each having three isotypes, α, β and γ. All-trans retinoic acid (ATRA) is the carboxylic acid form of vitamin A and its major metabolite. The actions of ATRA are generally mediated by binding to RARs, which act as ligand-regulated transcription factors by binding as heterodimers with the RXRs to ATRA response elements located in regulatory regions of target genes (Thacher et al., 2000). On the other hand, retinoids have been known to affect the activities of various protein kinases, including protein kinase C (PKC) (Aggarwal et al., 2006), protein kinase A (PKA) (Zhao et al., 2004) and mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase 1/2 (ERK1/2) (Hong et al., 2001; Canon et al., 2004), p38 (Alsayed et al., 2001) and Jun N-terminal kinase (JNK) (Lee et al., 1999; Moreno-Manzano et al., 1999). There is increasing evidence that ATRA can induce rapid extragenomic effects that stimulate kinase signalling pathways (Canon et al., 2004; Aggarwal et al., 2006) by mechanisms still not yet clear, which are independent of receptor binding to DNA response elements.
Retinoids were shown to have excellent preventive and therapeutic effects in various experimental kidney diseases (reviewed by Xu et al., 2004). Among the renal cells, we have recently identified glomerular mesangial cells (MC) as a target for the anti-inflammatory effects of retinoids (Moreno-Manzano et al., 2000). MC line the blood vessels of the renal glomerulus and play an important role in proliferative glomerulonephritis (Pfeilschifter, 1994). In anti-Thy 1.1 nephritis, a rat model of mesangial proliferative glomerulonephritis, ATRA has very good therapeutic effects (Wagner et al., 2000). Recently, our preliminary results suggested that ATRA increased the expression of COX-2 and the production of PGE2 in MC. These preliminary results are particularly interesting because, although it is traditionally believed that COX-2 is a proinflammatory enzyme, its increased expression, associated with increased production of PGE2 in MC, could be a protective response; PGE2, once considered a proinflammatory molecule by virtue of its vasodilator and nociceptive properties, also displays inhibitory effects on most leucocyte functions. Nowadays, it is considered a modulator of inflammation rather than a strictly proinflammatory mediator (Tilley et al., 2001). In MC, expression of COX-2 and production of PGE2 are increased in response to inflammatory cytokines such as IL-1β (Soler et al., 2001) and it was also shown that PGE2 inhibits MC proliferation (Stahl et al., 1990). Interestingly, overexpression of COX-2 also exerts antiproliferative effects on MC by a mechanism independent of PG synthesis (Zahner et al., 2002).
The aim of the present work was to confirm that ATRA increased mesangial expression of COX-2 and to examine the mechanisms involved in this effect. We found that ATRA upregulated not only the expression of COX-2 and COX-1 but also increased the production of PGE2. Regarding the mechanism involved in COX-2 upregulation, ATRA acts at the transcriptional, but not the post-transcriptional, level of COX-2 expression and is mediated by ERK1/2. Interestingly, RARs were not required for the upregulation of COX-2. These results indicate that ATRA is an important regulator of the mesangial expression of COX isoenzymes and highlight the importance of kinase-dependent and RAR-independent mechanisms in the effect of ATRA.
Methods
Cell culture
Human MC were obtained from adult specimens, as we described previously (Moreno-Manzano et al., 2000). The identity of the cells was confirmed by morphologic and functional criteria. Under phase-contrast microscopy, all cells appeared large and stellate and no cells with epithelial or endothelial morphologic characteristics were seen. MC showed histochemical evidence of containing actomyosin fibres, and they did not stain for factor VIII, unlike endothelial cells. In addition, all cells examined contracted after incubation with platelet-activating factor, angiotensin II and arginine-vasopressin. The culture medium was Roswell Park Memorial Institute 1640 supplemented with 10% foetal bovine serum (FBS), 20 mM L-glutamine and antibiotics (penicillin 100 U ml−1 and streptomycin 100 μg ml−1). Confluent cells between 6th and 15th passages were used and they were made quiescent when appropriate by 24 h incubation with medium supplemented with 0.5% FBS.
Transient transfection and luciferase assay
A total of 3.5 × 105 cells/well were plated in six-well plates 24 h before transfection. The cells in every well were then incubated 8 h at 37°C with 2 ml Opti-MEM (Invitrogen, CA, USA) containing complexes of 5 μg LipofectAMINE (Invitrogen, CA, USA), 1.0 μg human COX-2 reporter and 0.1 μg renilla luciferase reporter as an internal control. Transfected cells were next incubated with complete growth medium for 16 h and then they were treated as indicated in the legend of Figure 4c. Finally, firefly luciferase activity of the COX-2 reporter was measured with a Lumat LB9506 luminometer (Berthold Technologies, Herts, UK) and normalized against the renilla luciferase activity by using the dual-luciferase reporter assay system (Promega, Madison, WI, USA).
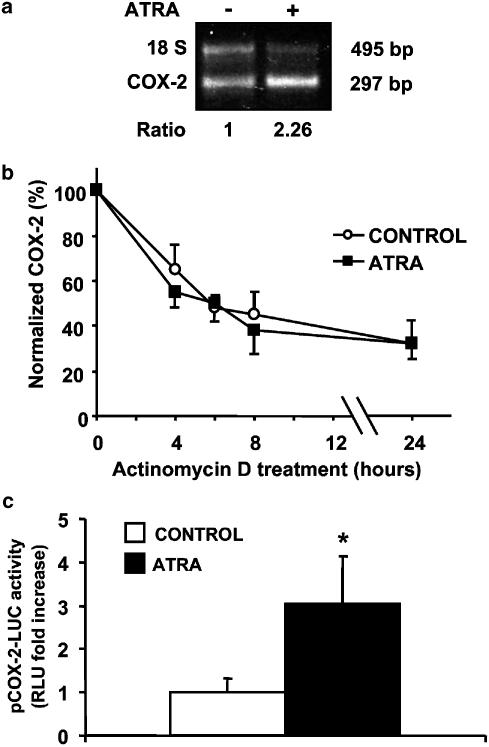
Figure 4.
ATRA increases COX-2 expression at the transcriptional level of regulation. (a) 10 μM ATRA increases the mesangial expression of COX-2 mRNA, analysed by semiquantitative RT-PCR, after a 24 h incubation. Equal mRNA loading was confirmed by coamplification of 18S mRNA. Normalized density ratio of COX-2 over 18 S RNA is indicated for each band. The photograph represents at least three repeated experiments. (b) ATRA-treated cells do not show a decreased decay rate of COX-2 mRNA as compared to controls. The time course of the decay of COX-2 mRNA was analysed by Northern blot, in MC treated for 24 h with ATRA vehicle or with 10 μM ATRA and then with 2 μg ml−1 actinomycin D for 0–8 h. Equal mRNA loading was confirmed by probing with GAPDH. The graph is the densitometric analysis of the COX-2 mRNA level normalized to the level of GAPDH mRNA. Results are the mean±s.d. of four separate experiments (c) ATRA (10 μM/24 h) increases the activity in MC of the human COX-2 gene promoter, determined in triplicate in four separate experiments, by transient transfection assay. *P<0.01 vs control.
RT–PCR analysis of COX-2 expression
Total RNA was extracted using the TriPure isolation reagent (Roche Diagnostics, GmbH, Mannheim, Germany) according to the manufacturer's instructions and spectrophotometrically quantified. The reverse transcriptase–polymerase chain reaction (RT–PCR) reaction was performed with the cMaster RTplusPCR system (Eppendorf AG, Hamburg, Germany) with specific primers for human COX-2 purchased to Ambion (Austin, TX, USA) ((F) 5′-CATTCTTTGCCCAGCACTTCAC-3′; (R) GACCAGGCACCAGACCAAAGAC; Accession number: D28235). Modified 18S primers (QuantumRNA 18S Internal Standards, Ambion) were used for 18S coamplification, as constitutive controls. The reaction mixture was incubated for 60 min at 42°C and 2 min at 94°C, followed by 28 cycles of 30 s at 94°C, 30 s at 59°C and 30 s at 72°C, with a final extension of 5 min at 72°C. Preliminary experiments established that these conditions provided a linear cDNA amplification. PCR products were separated on 2% agarose gels, and bands were visualized by ethidium bromide staining.
Northern blot analysis
Total RNA was extracted by a single-step method with TriPure isolation reagent (Roche Diagnostics, GmbH, Mannheim, Germany) following the manufacturer's protocol and subjected to Northern blot analysis, as described previously (Kitamura, 1997). In brief, RNA samples were electrophoresed on 1.2% agarose gels containing 10% formaldehyde and transferred onto nitrocellulose membranes. A human COX-2 cDNA (Cayman Chemical, Ann Arbor, MI, USA) and a human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA were used as probes and labelled with 32P-dCTP using the random priming method. The membranes were hybridized with the probes at 65°C overnight in a solution containing 4 × saline sodium citrate (600 mM NaCl, 60 mM sodium citrate), 5 × Denhardt's solution, 10% dextran sulphate, 50 μg ml−1 Herring sperm DNA and 50 μ ml−1 poly(A), washed at room temperature and exposed to X-ray films at −80°C. The intensity of mRNA was evaluated quantitatively by densitometric analysis.
Western blot analysis
Cells were homogenized in a solution containing 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 5 mM ethylene di amine tetra acetate, 1% deoxycholic acid, 0.1% sodium do decyl sulphate, 1% Triton X-100 and protease inhibitors 1 mM phenylmethylsulphonylfluoride, 10 μg ml−1 aprotinin, 2 μg ml−1 leupeptin and the phosphatase inhibitor 0.2 mM NaVO4. Cell proteins (30–40 μg) were run in an 8–10% SDS–polyacrylamide gel, transferred onto a nitrocellulose membrane (Trans-Blot Transfer Medium, Bio-Rad, CA, USA) and incubated overnight at 4°C with antibodies recognizing specifically COX-1, COX-2, RAR-β, P-ERK, P-CREB (cyclic AMP-response-element-binding protein) as described previously (Romero-Sandoval et al., 2004). This incubation was followed by a second incubation with a peroxidase-conjugated secondary antibody and immunoreactive products were detected by chemiluminiscence using the enhanced chemiluminescence Western Blotting Detection Reagents (Amersham Biosciences, UK) following the protocol provided by the manufacturer. As a loading control, blots probed with anti-COX-1, anti-COX-2 and anti-RAR-β were subsequently re-probed with anti-α-actin, whereas blots probed with anti-P-ERK, anti-P-CREB and anti-P-mitogen- and stress-activated protein kinase-1 (MSK1) were re-probed with anti-total ERK2, anti-total CREB or anti-α-actin, respectively.
Determination of PGE2 formation
The culture medium of MC grown in six-well plates and treated as described in the legends of Figures 1c and 2a was collected and diluted two times. PGE2 in the medium was determined in triplicate using an enzyme immunoabsorbent assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) following the manufacturer's protocol. The assay was performed in a total volume of 150 μl, with the following components being added in 50-μl volumes: standards or biological samples, enzymatic tracer and specific antiserum. After overnight incubation at 4°C, the plates were washed, and 200 μl Ellman's reagent was added into each well. After 1–2 h, the absorbance at 414 nm of each well was measured. A standard curve from 50 to 0.39 pg ml−1 was used to evaluate the concentrations of PGE2. Results were calculated by using the non-linear regression of a four-parameter logistic model.
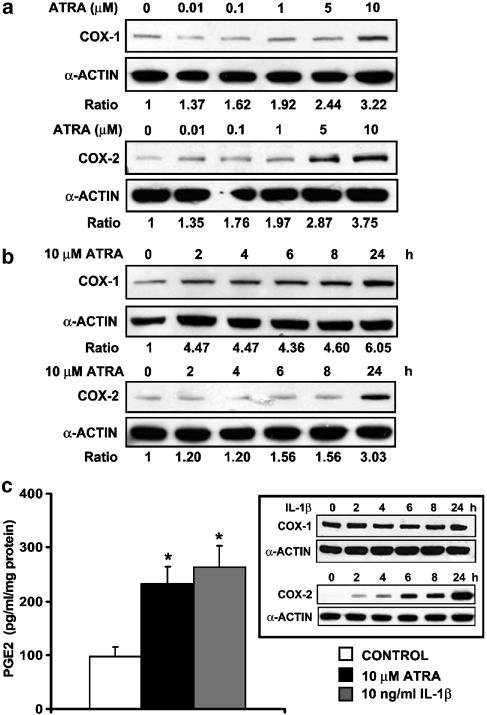
Figure 1.
ATRA upregulates COX-1 and COX-2 protein expression and increases PGE2 production in MC (a, b). Expression of COX-1 and COX-2 proteins was analysed by Western blot in MC (a) incubated for 24 h with the indicated concentrations of ATRA or (b) incubated with 10 μM ATRA for the indicated times. Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of either COX-2 or COX-1 over α-actin is indicated for each band. Each photograph represents at least three repeated experiments. (c) PGE2 production in MC incubated for 24 h with 10 μM ATRA. PGE2 in the medium was determined in triplicate in four separate experiments. For comparison, the effects of 10 ng ml−1 IL-1β on PGE2 production as well as on the expression of COX-1 and COX-2 (inset) were analysed in parallel. *P<0.01 vs control.
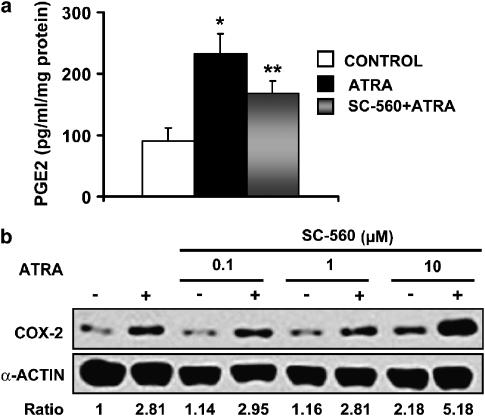
Figure 2.
Effect of the inhibition of COX-1 with SC-560 on PGE2 production and COX-2 upregulation in ATRA-treated MC. Cells were preincubated for 1 h with 100 nM SC-560 and then for 24 h with 10 μM ATRA. (a) PGE2 production, determined in triplicate in four separate experiments. *P<0.01 vs control, **P<0.05 vs control and ATRA groups. (b) Expression of COX-2 protein (Western blot analysis). Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of COX-2 over α-actin is indicated for each band. The photograph represents at least three repeated experiments.
Data analysis and statistical procedures
All values are presented as means±s.d.. All experiments were repeated a minimum of three times. Statistical significance between individual groups was tested using the non-parametric unpaired Mann–Whitney U-test. A P-value of <0.05 was considered significant.
Drugs and chemical reagents
The RARs pan-agonists ATRA and TTNPB ([4-(E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl] benzoic acid) were purchased from Sigma (St Louis, MO, USA) and Tocris (Ellisville, MO, USA), respectively. The selective RAR pan-antagonists LE540 and AGN193109 were kindly provided by Dr H Kagechika (School of Biomedical Science, Tokyo Medical and Dental University, Tokyo, Japan) and Dr RAS Chandrararatna (Allergan, Irvine, CA, USA), respectively. Actinomycin D and cycloheximide, respective inhibitors of RNA and protein synthesis, were purchased from Sigma (St Louis, MO USA). The kinase inhibitors PD98059 and U0126 (MAPK kinase-1 (MKK1) inhibitors), SB203580 (p38 MAPK inhibitor), SP600125 (JNK MAPK inhibitor), GF 109203X (p90 ribosomal S6 kinase (RSK2) inhibitor) and H89 (MSK1 inhibitor) were purchased from Calbiochem (La Jolla, CA, USA). PKA inhibitor fragments 14–22 myristoylated (PKA inhibitor) was purchased from Sigma (St Louis, MO, USA). [9-(tetrahydro-2′-furyl) adenine] (or SQ22536, inhibitor of adenylyl cyclase), IL-1β and the COX-1-selective inhibitor SC-560 were purchased, respectively, from Calbiochem (La Jolla, CA, USA), Roche (Indianapolis, IN, USA) and Cayman Chemical (Ann Arbor, MI, USA). All reagents were prepared in dimethyl sulphoxide so that the final concentration was <0.1%, except PKA inhibitor, actinomycin D, cycloheximide and IL-1β which, were dissolved in sterile water. The human COX-2 luciferase reporter construct phPES2 containing the promoter fragments −327 to +59 (Inoue et al., 1995) was a gift from Dr Hiroyasu Inoue (Nara Women's University, Nara, Japan). Primary antibodies against COX-2, RAR-β and total ERK2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Primary antibody against COX-1 was obtained from both Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Cayman Chemical Company, (Ann Arbor, MI, USA); antibodies against total CREB and against the phosphorylated forms of ERK1/2 (P-ERK1/2), MSK-1 (P-MSK1) and CREB (P-CREB) were purchased from Cell Signaling Technology (Beverly, MA, USA) and an anti-α-actin antibody was from Sigma Chemical Co. (St Louis, MO, USA). All antibodies were used at a 1:1000 dilution.
Results
ATRA upregulates the expression of COX-1 and COX-2 proteins and production of PGE2 in MC
Expression of COX-1 and COX-2 in unstimulated and ATRA-stimulated MC was examined. Serum-deprived cultured MC constitutively expresses both COX-1 and COX-2 in the absence of stimulation. ATRA treatment for 24 h dose-dependently induced COX-2 protein expression, which was evident at ATRA concentrations as low as 0.01 μM (Figure 1a). The maximum effect was observed at concentrations higher than 5 μM. Equal protein loading was confirmed by probing with an anti-α-actin antibody. ATRA also elicited an increase in the mesangial levels of COX-1, but, in contrast with COX-2, only the highest concentration tested, 10 μM, had an effect on human MC. Given that an increase in the levels of mesangial COX-1 is rather unusual, but possible (Hartner et al., 2000), we repeated these experiments using an antibody against COX-1 obtained from another commercial source (Cayman Chemical Company, Ann Arbor, MI, USA) and similar results were obtained (data are not shown).
The effects of ATRA on the levels of COX-1 and COX-2 protein were examined further using different incubation times. MC were treated with ATRA (10 μM) for up to 24 h and Western blot analysis was performed. As shown in Figure 1b, the COX-1 protein levels increased early after the treatment with ATRA, modestly after 2 h and markedly after 24 h (Figure 1b), whereas an increased COX-2 was found only after a 24 h incubation with the retinoid.
We next confirmed that upregulation of COX protein expression was followed by increased production of PGE2. As shown in Figure 1c, when cells were incubated with ATRA for 24 h, there was an increase in the amount of PGE2 released into the medium relative to vehicle-treated cells, as measured by specific enzyme immunoassay. PGE2 released from cells incubated with IL-1β, a strong inducer of COX-2, but not of COX-1 (Figure 1c, inset), exhibited an increase of PGE2, similar to that observed in ATRA-stimulated MC. The concentration of IL-1β used was chosen on the basis of previous dose–response experiments to obtain a maximal effect. These results suggest that the potency of ATRA as an inducer of PG synthesis is comparable to that of classical inducers such as IL-1β.
COX-1-dependent synthesis of PGs is not involved in ATRA-induced increase of COX-2 protein expression
Both COX isoforms may be upregulated by their products (Maldve et al., 2000). Given that the expression levels of COX-1 increased earlier than those of COX-2 after ATRA treatment (Figure 1b), there was a possibility that PGs synthesized by COX-1 were responsible for ATRA-induced COX-2. This possibility was investigated by selective inhibition of COX-1 with SC-560, before ATRA treatment. The IC50 value for SC-560 with respect to COX-1 is 9 nM, whereas the corresponding IC50 value for COX-2 is 6.3 μM (Smith et al., 1998). We incubated MC with 100 nM SC-560, a concentration well above the IC50 value for COX-1 but still well below the IC50 value for COX-2, and found that SC-560 pretreatment significantly inhibited the increased release of PGE2 into the culture medium of ATRA-treated cells (Figure 2a). Furthermore, dose–response experiments showed that pre-incubation with SC-560 (range 0–10 μM) did not inhibit ATRA-induced COX-2 expression (Figure 2b). Actually, pretreatment with 10 μM SC-560 synergized with ATRA to induce a higher increase in COX-2 expression, and it also increased basal COX-2 expression. This is probably because of the IC50 value for COX-2 inhibition, which is 6.3 μM, has been reached: in our experience, when COX-2 activity is inhibited (for instance, with celecoxib), basal COX-2 protein expression results upregulated and there is a synergic effect with ATRA to upregulate COX-2 expression (unpublished data from our laboratory). In summary, these results rule out the possibility that COX-1-dependent synthesis of PGs is the main mechanism involved in ATRA-induced increase of COX-2 protein expression.
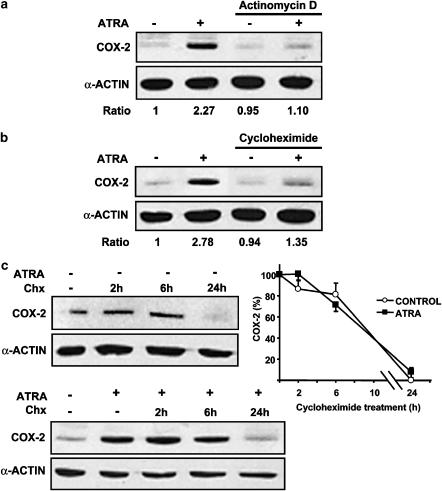
ATRA-induced increase of COX-2 protein expression is inhibited by actinomycin D and cycloheximide and not owing to increased stability of COX-2 protein
To clarify whether the enhancement by ATRA of COX-2 expression was owing to de novo synthesis or not, we examined the effects of actinomycin D, an inhibitor of transcription, and the effect of cycloheximide, an inhibitor of protein synthesis. As shown in Figure 3, preincubation of MC with either 2 μg ml−1 actinomycin D (Figure 3a) or 10 μM cycloheximide (Figure 3b) resulted in an inhibition of ATRA-induced increase of COX-2 protein expression as compared to cells preincubated with vehicle.
Figure 3.
ATRA-induced increase of COX-2 protein expression is inhibited by actinomycin D and cycloheximide and not owing to increased stability of COX-2 protein. Expression of COX-2 protein after 24 h incubation with ATRA vehicle in control conditions or with 10 μM ATRA was analysed by Western blot in MC. (a) Preincubated for 1 h in control conditions or with 2 μg/ml actinomycin D or (b) preincubated for 1 h in control conditions or with 10 μM cycloheximide or (c) incubated for 0–24 h with 10 μM cycloheximide (Chx) to analyse the stability of COX-2 protein. Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of COX-2 over α-actin is indicated for each band. Each photograph represents at least three repeated experiments. The graph in panel c represents the COX-2 protein levels normalized to α-actin. Results from the densitometric analysis were expressed as arbitrary values and plotted relative to the zero time point. The protein stability was similar between control and ATRA-treated cells, the half-life being <9 h in both groups.
We also studied whether ATRA increases the stability of COX-2 protein. MC were incubated for 24 h with either ATRA vehicle (Figure 3c, upper panel) or 10 μM ATRA (Figure 3c, lower panel). Afterwards, cells were incubated for 0–24 h with 10 μM cycloheximide, and Western blot analysis of COX-2 protein showed no difference in the rate of COX-2 degradation, regardless whether cells were incubated or not with ATRA.
Increased COX-2 expression by ATRA is predominantly owing to transcriptional regulation
Expression of COX-2 mRNA in unstimulated and ATRA-stimulated MC was examined. Serum-deprived human MC were treated with or without ATRA for 24 h, and semiquantitative RT–PCR was performed. MC expressed a low level of basal COX-2 mRNA, and the expression was substantially upregulated by the treatment with ATRA (Figure 4a).
In other cell systems, alterations in COX-2 mRNA expression were attributed to both transcriptional and post-transcriptional regulation. To test the possibility that there was a component of post-transcriptional stabilization of COX-2 mRNA in response to ATRA, MC were treated for 24 h with ATRA vehicle or with ATRA and subsequently chased the decay of COX-2 mRNA after the addition of actinomycin D for 0–8 h. We analysed the decay of COX-2 mRNA by Northern blotting. The time course in ATRA-treated cells did not show a decreased decay rate of COX-2 mRNA as compared to controls (Figure 4b), suggesting that ATRA does not regulate COX-2 mRNA stability.
To examine whether ATRA at 24 h can induce transcription from the COX-2 promoter, we used pHPES2 (−327/+59), a plasmid that expresses firefly luciferase under the control of the human COX-2 gene promoter (−327/+59). Transient transfection assay showed that ATRA increased the activity of the human COX-2 gene promoter (Figure 4c), which is consistent with the upregulation of the expression of COX-2 mRNA by ATRA.
In summary, the data shown in this section indicate that increased COX-2 expression by ATRA is predominantly owing to transcriptional regulation.
Pharmacological antagonists of RAR and RXR do not have an effect on ATRA-induced increase of COX-2 protein expression and a pharmacological agonist of RAR does not upregulate COX-2
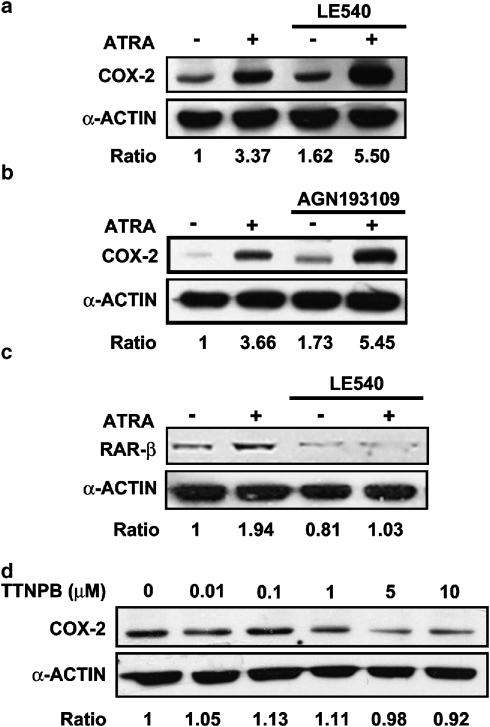
The effects of a RAR pan-antagonist LE540 in the regulation of COX-2 by ATRA was examined by treating MC with the antagonist for 1 h and then stimulating with ATRA. Western blot analysis showed that the induction of COX-2 by ATRA was unaffected by this antagonist (Figure 5a). We repeated the experiment using a different RAR pan-antagonist, AGN193109. Again Western blot analysis showed no inhibitory effect of the RAR pan-antagonist on ATRA-induced increase of COX-2 protein expression (Figure 5b), although it inhibited a typical RAR-dependent effect, such as the upregulation of RAR-β expression (Thacher et al., 2000) by ATRA (Figure 5c).
Figure 5.
Effect of pharmacological modulation of retinoic acid receptors (RARs) in MC. Western blot analysis of (a,b) the expression of COX-2 protein after 24 h incubation with vehicle or with 10 μM ATRA in cells pretreated for 1 h with (a) RAR pan-antagonist LE540 (2.5 μM) or (b) RAR pan-antagonist AGN193109 (2.5 μM). (c) The expression of RARβ protein after 24 h incubation with vehicle or with 10 μM ATRA in MC pretreated for 1 h with either RAR pan-antagonist LE540 (2.5 μM). (d) The expression of COX-2 protein after 24 h incubation with the RAR pan-agonist TTNPB. Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of COX-2 or RARβ over α-actin is indicated for each band. The photograph represents at least three repeated experiments.
Furthermore, incubation of cells with the stilbene-based retinoid TTNPB, a RAR pan-agonist (Thacher et al., 2000), did not result in an increased COX-2 expression (Figure 5d). In summary, these results indicate that COX-2 expression induced by ATRA is through a mechanism independent of RAR.
Phosphorylation of ERK1/2 and downstream MSK1 and CREB in ATRA-treated cells: role of ERK1/2 in the ATRA-induced increase of COX-2 protein expression
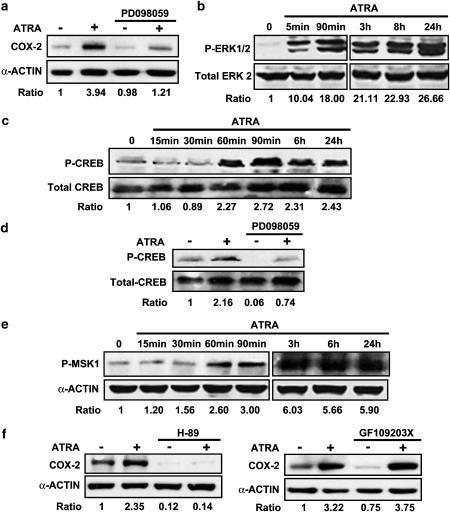
We and others have reported that ATRA enhances ERK1/2 (Hong et al., 2001; Xu et al., 2002). Induction of COX-2 in MC in response to external stimuli is mediated by several signalling molecules including ERK1/2 (Sawano et al., 2002). Therefore, the stimulatory effect of ATRA on COX-2 could be mediated by ERK1/2. To examine this possibility, serum-deprived MC were treated for 1 h with the selective inhibitor of MKK1 PD098059 and then stimulated with ATRA. Western blot analysis showed that ATRA-inducible expression of COX-2 was greatly attenuated by the treatment with PD098059. (Figure 6a). Similar results were found when the experiment was repeated with another inhibitor of MKK1, U0126 (results are not shown). To further analyse the involvement of ERK1/2 in the ATRA-induced increase of COX-2 activity, we examined the effects of ATRA on ERK1/2. Serum-deprived MC were stimulated with ATRA for 0–24 h, and kinase phosphorylation was evaluated. Under the basal culture condition, MC exhibited modest ERK phosphorylation. It was quickly and significantly increased by the treatment with ATRA (Figure 6b).
Figure 6.
Phosphorylation of ERK 1/2 and downstream MSK1 and CREB in ATRA-treated cells: role of ERK1/2 in the ATRA-induced increase of COX-2 protein expression. (a) Effect of preincubation with the MKK1 inhibitor PD098059 (50 μM/1 h) on the upregulation of COX-2 by ATRA (10 μM/24 h). Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of COX-2 over α-actin is indicated for each band. (b, c) Time course of the phosphorylation of ERK1/2 (P-ERK1/2) (B) and CREB (P-CREB) (c) following incubation with 10 μM ATRA. Equal protein loading was confirmed by probing with anti-total ERK2 or anti-total CREB antibodies, respectively. Normalized density ratio of ERK1/2 over total ERK2 or of P-CREB over total CREB is indicated for each band. (d) Effect of pre-incubation with the MKK1 inhibitor PD098059 (50 μM/1 h) on the phosphorylation of CREB (P-CREB) by ATRA 10 μM/24 h). Equal protein loading was confirmed by probing with anti-total CREB antibody. Normalized density ratio of P-CREB over total CREB is indicated for each band. (e) Phosphorylation of MSK1 (P-MSK1) following incubation with 10 μM ATRA. Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of P-MSK1 over α-actin is indicated for each band. (f) Effect of preincubation with the MSK-1 inhibitor H89 or the ERK1/2-activated ribosomal proteinS6 kinase-2 (RSK2) inhibitor GF109203X (10 μM each/1 h) on the upregulation of COX-2 by ATRA (10 μM/24 h). Equal protein loading was confirmed by probing with an anti-α-actin antibody. Normalized density ratio of COX-2 over α-actin is indicated for each band. Every photograph represents at least three repeated experiments.
The nuclear transcription factor CREB is a major downstream target of ERK1/2 that initiates the transcription of the human COX-2 gene (Xie et al., 1994). It has been recently found that CREB may be phosphorylated by ATRA following the activation of ERK1/2 by ‘non-genomic' mechanisms (Canon et al, 2004; Aggarwal et al., 2006). In MC, ATRA had similar effects as CREB was also phosphorylated following treatment with ATRA (Figure 6c) and prevention of ATRA-induced activation of ERK1/2 by preincubation with PD098059 resulted in a great attenuation of both CREB phosphorylation (Figure 6d) and COX-2 expression (Figure 6a). As these data suggested that activation of CREB following the activation of ERK1/2 may be relevant for ATRA-induced COX-2 upregulation, we studied the events upstream of CREB. CREB is not directly phosphorylated by ERK1/2: it is phosphorylated and transactivated by ERK1/2-activated ribosomal S6 kinases (RSKs) and mitogen- and stress-activated protein kinases (MSKs) (Deak et al., 1998; Frodin and Gammeltoft, 1999). Western blot analysis showed that ATRA-induced MSK1 phosphorylation (Figure 6e) and that pretreatment with the MSK1 inhibitor H89 (Vermeulen et al., 2003) abolished both basal- and ATRA-induced expression of COX-2, whereas pretreatment with the RSK2 inhibitor GF 109203X (Alessi, 1997) did not have any effect on the upregulation of COX-2 by ATRA (Figure 6f).
Other studies on kinases
The p38 MAPK inhibitor SB203580 and the JNK MAPK inhibitor SP600125 had no effect on the basal- and ATRA-induced COX-2 (Supplementary Figure 1a).
Phosphorylation of CREB at Ser133 may be induced by agonists that elevate the intracellular concentration of cyclic AMP, with ATRA being among them (Zhao et al., 2004), through PKA (cyclic AMP-dependent protein kinase). As H89 inhibits PKA (Davies et al, 2000) and PKA has been described as an upstream activator of ERK-1/2 (Stork and Schmitt, 2002) and MSK1 (Delghandi et al., 2005), it is conceivable that activation of PKA by ATRA may be involved in ATRA-induced COX-2 upregulation. To test this possibility, we studied the effect of the inhibition of PKA by preincubation with either the PKA inhibitor fragments 14-22 myristoylated or the adenylate cyclase inhibitor SQ22536 on ATRA-induced COX-2 expression. Our results indicated that preincubation with these inhibitors did not have any effect on the induction of COX-2 by ATRA (Supplementary Figure 1b).
Discussion and conclusions
The aim of the present work was to confirm that ATRA increased mesangial expression of COX-2 and to examine the mechanisms involved in this effect. We found that ATRA upregulates not only the expression of COX-2 but also that of COX-1. In ATRA-stimulated MC, the production of PGE2, the most important prostanoid released by MC after stimulation with IL-1β (Soler et al., 2001), increased to an extent similar to those found in IL-1β-stimulated cells. However, the contribution of COX isoenzymes to PGE2 production was different between the two stimuli: IL-1β induced COX-2 but not COX-1, whereas ATRA upregulated both isoenzymes (Figure 1). Two additional targets of ATRA may be phospholipase A2, responsible for the release of arachidonic acid (the major prostanoid precursor) from phospholipids, and the membrane-associated PGE synthase that converts PGH2 to PGE2 (Smith et al., 2000). It has been shown that 9-cis retinoic acid induces both enzymes (Tsukamoto et al., 2004), although there was no effect on PGE2 production, probably because there was no associated increase in COX expression. In fact, PGE2 production increased dramatically when expression of COX-2 was increased by lipopolysaccharide. Given that the potency of ATRA as an inducer of PGE2 synthesis in MC is comparable to that of classical inducers such as IL-1β, further experiments should be performed to analyse the potential contribution of increased expression of phospholipase A2 and PGE synthase to the effect of ATRA on PGE2 production.
The increase in mesangial COX-2 expression was mainly owing to a transcriptional effect of ATRA, as indicated by the increased expression of COX-2 mRNA. This was found to be owing to the increased activity of the COX-2 promoter and not to the changes in the stability of COX-2 mRNA or COX-2 protein (Figures 3 and 4). We also excluded the possibility that PGs synthesized by COX-1 were responsible for ATRA-induced upregulation of COX-2 as inhibition of COX-1 activity by preincubation with SC-560 did not inhibit the effect of ATRA on the expression of COX-2 (Figure 2).
We have previously shown that treatment with ATRA results in an increased COX-2 expression in rat spinal cord (Romero-Sandoval et al., 2004). ATRA or its stereoisomer 9-cis retinoic acid induced neither COX-2 nor PGE2 in PMA-differentiated U937 cells (Tsukamoto et al., 2004). In another study, ATRA repressed COX-2 promoter activity and COX-2 mRNA expression in several murine lung tumour-derived cell lines, yet increased promoter activity and COX-2 mRNA expression significantly in another lung tumour-derived cell line (Wardlaw et al., 2002). These data indicate that the effect of ATRA on COX-2 expression is likely to be cell specific.
The importance of MAPKs in the induction of COX-2 under various situations is very well known. The family of MAPKs includes the ERK1/2, JNK and p38 kinases (Schaeffer and Weber, 1999) and the three members of this family have been previously shown to be involved in the generation of retinoid responses. In general, ATRA is well known as an inhibitor of JNK (Lee et al., 1999; Moreno-Manzano et al., 1999). In contrast, it was reported that ATRA activates p38 MAPK and ERK1/2 (Alsayed et al., 2001, Hong et al., 2001). Consistent with these data, we have shown in MC that ATRA inhibits JNK MAPK (Moreno-Manzano et al., 1999) whereas it activates p38 MAPK (unpublished observations) and ERK1/2 (Xu et al., 2002). Therefore, the stimulatory effect of ATRA on COX-2 could be mediated by ERK1/2 and/or p38 MAPK. The pharmacological inhibition of these pathways (Figure 6a and Supplementary Figure 1a) suggested that ERK1/2, but not p38 MAPK, was relevant to the effect of ATRA as an attenuation of ATRA-induced COX-2 expression was only achieved by inhibition of ERK1/2 activation through MKK1 inhibition (Figure 6a). An important downstream event of the ERK1/2 pathway is the phosphorylation of the transcription factor CREB, which is known to activate COX-2 transcription (Juttner et al., 2003). CREB is not directly phosphorylated by ERK1/2: there are two kinases targeted by ERK1/2, namely RSK2 and MSK1, that directly phosphorylate CREB (Deak et al., 1998; Frodin and Gammeltoft, 1999). Treatment of MC with ATRA resulted in increased phosphorylation of CREB (Figure 6c) and MSK1 (Figure 6e), and pretreatment with the MKK1 inhibitor PD098059 resulted in the simultaneous inhibition of CREB phosphorylation and COX-2 upregulation (Figure 6a and d). Inhibition of MSK-1, but not of RSK-2, also resulted in the abolition of ATRA-induced COX-2 upregulation (Figure 6f). These data suggest that activation of MSK-1 and CREB following activation of ERK1/2 might be relevant for ATRA-induced COX-2 upregulation, although genetic deletion/inhibition of these key molecules is needed to strengthen this argument. Interestingly, although ERK1/2 was clearly phosphorylated within the first hour after ATRA treatment, there was a delay of 24 h before the upregulation of COX-2 by the retinoid were evident. This raises the possibility that some other molecules or signalling cascades might be involved between the early initiation of ERK1/2 activation and the phase of sustained activation of ERK 1/2 leading to increased COX-2 expression.
The human COX-2 promoter region (−327/+59) contains 3 cis-acting elements, namely, a nuclear factor-κB binding site a nuclear factor-IL 6 binding site, and a cyclic AMP response element, all of which have been shown to be involved in the regulation of COX-2 gene transcription. Therefore, they are the potential final targets for the signalling cascade leading to increased COX-2 expression after treatment with ATRA. The transcriptional effects of ATRA are most commonly mediated by binding to nuclear receptors RARs, which normally act as ligand-inducible transcription factors by binding as heterodimers with the RXRs to DNA response elements known as retinoic acid response elements (Thacher et al., 2000). As there are no retinoic acid response elements in the promoter, ATRA-activated RAR–RXR heterodimers are not expected to be directly involved in a direct activation of the COX-2 promoter. It is also unlikely that ATRA-activated RAR–RXR heterodimers may participate in any step of the mechanism through which ATRA upregulates COX-2 expression in MC. This is because TTNPB, which is widely used to define RAR-specific effects, did not upregulate mesangial COX-2 and the pharmacologic inhibition of RARs did not result in the inhibition of the effect of ATRA on COX-2 expression (Figure 5).
In summary, we do not know which molecule ATRA targets in order to initiate the signalling events that leads to ERK1/2 phosphorylation and, finally, to COX-2 upregulation. Early induction of ERK1/2 phosphorylation by ATRA in MC is very rapid and is an additional evidence that ATRA can have in MC effects non-mediated by nuclear receptors RAR and RXR. In fact, rapid ‘non-genomic' effects of ERK1/2 phosphorylation induced by ATRA, resulting in CREB phosphorylation as in our experiments, have been already described in neuronal (Canon et al., 2004) and bronchial epithelial cells (Aggarwal et al., 2006). Activation of ERK1/2 may be owing to an unknown receptor for ATRA or to a direct interaction of ATRA with upstream components of the signalling pathway for ERK1/2 activation. However, to reiterate, this mechanism by which ATRA can cause ERK1/2 activation, a crucial event for the upregulation of mesangial COX-2, is still unknown. Further extensive studies are needed to discover which molecule ATRA targets that initiates this signalling event in MC.
The in vivo significance of the upregulation of both COX isoenzymes by ATRA may be related to the inhibitory effect of ATRA on glomerular inflammation. One may speculate that upregulation of both COX isoenzymes could play a significant role in the anti-inflammatory effects of ATRA in the glomerulus. This is supported by the report that COX-induced PGs inhibited the proinflammatory responses of MC (Stahl et al., 1990). In addition, in mesangial proliferative glomerulonephritis, inhibition of COX-1 and COX-2 exacerbated infiltration of monocytes/macrophages (Schneider et al., 1999) much more than preferential COX-2 inhibition. However, we also appreciate that COX-2 has dual functions in different phases of inflammation, as inhibition of COX-2 in the induction phase of inflammation suppresses inflammation, and inhibition of COX-2 in the resolving phase prevents recovery (Gilroy et al., 1999). It is therefore possible that the biological/pharmacological effects of ATRA-induced COX-2 may vary with the clinical phase of the inflammation.
In conclusion, in our experiments we showed for the first time that ATRA upregulated COX-2, as well as COX-1, and PGE2 biosynthesis in human MC. Regarding the underlying mechanism of COX-2 upregulation, we found that ATRA acts at the transcriptional level, but not at the post-transcriptional level. The signal cascade following ATRA treatment involves the activation of ERK1/2, without the participation of nuclear receptors RAR- nor COX-1-dependent synthesis of PGs. These results indicate that ATRA is an important regulator of the mesangial expression of COX isoenzymes and highlight the importance of kinase-dependent and RAR-independent mechanisms in the effect of ATRA.
Conflicts of interest
The authors state no conflict of interest.
External data objects
Acknowledgments
This work was supported by grants from FISS PI020349 the Spanish Ministry of Education and Science (SAF2005-06242-C03-01), the Comunidad de Madrid-Universidad de Alcala (CAM-UAH2005/001) and the Fundación Médica Mutua Madrileña Automovilista. M Alique is a fellow of the Spanish Ministry of Education and Science. We thank Mr L Baron for his help with the English revision. We thank Dr RAS. Chandraratna (Allergan, Irvine, CA, USA) for the kind gift of AGN193109; Dr H Kagechika (Tokyo Medical and Dental University, Tokyo, Japan) for LE135 and Dr H Inoue (Nara Women's University, Nara, Japan) for the human COX-2 luciferase reporter construct phPES2.
Abbreviations
- ATRA
all-trans retinoic acid
- CREB
cyclic AMP-response-element-binding protein
- ERK1/2
extracellular signal-regulated kinase 1/2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- JNK
Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MKK1
MAPK kinase-1
- MC
mesangial cells
- MSK1
mitogen- and stress-activated protein kinase-1
- PG
prostaglandin
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PKC
protein kinase C
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- RSK2
p90 ribosomal S6 kinase
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Aggarwal S, Kim SW, Cheon K, Tabassam F, Joon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the camp response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR. The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997;402:121–123. doi: 10.1016/s0014-5793(96)01510-4. [DOI] [PubMed] [Google Scholar]
- Alsayed Y, Uddin S, Mahmud N, Lekmine F, Kalvakolanu DV, Minucci S, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J Biol Chem. 2001;276:4012–4019. doi: 10.1074/jbc.M007431200. [DOI] [PubMed] [Google Scholar]
- Canon E, Cosgaya JM, Scsucova S, Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell. 2004;15:5583–5592. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase (MSK-1) is directly activated by MAP and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH3T3 cells. Cell Signal. 2005;17:1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft RS. Role and regulation of 90 kDa ribosomal R6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-nash PR, Willis D, Chivers J, Paul-clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Hartner A, Pahl A, Brune K, Goppelt-Struebe M. Upregulation of cyclooxygenase-1 and the PGE2 receptor EP2 in rat and human mesangioproliferative glomerulonephritis. Inflamm Res. 2000;49:345–354. doi: 10.1007/PL00000215. [DOI] [PubMed] [Google Scholar]
- Hong HY, Varvayanis S, Yen A. Retinoic acid causes MEK-dependent RAF phosphorylation through RARalpha plus RXR activation in HL-60 cells. Differentiation. 2001;68:55–66. doi: 10.1046/j.1432-0436.2001.068001055.x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- Juttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, et al. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol. 2003;5:821–834. doi: 10.1046/j.1462-5822.2003.00324.x. [DOI] [PubMed] [Google Scholar]
- Kitamura M. Identification of an inhibitor targeting macrophage production of monocyte chemoattractant protein-1 as TGF-β1. J Immunol. 1997;159:1404–1411. [PubMed] [Google Scholar]
- Lee HY, Sueoka N, Hong WK Mangelsdorf DJ, Claret FX, Kurie JM. All-trans-retinoic acid inhibits Jun N-terminal kinase by increasing dual-specificity phosphatase activity. Mol Cell Biol. 1999;19:1973–1980. doi: 10.1128/mcb.19.3.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve RE, Kim Y, Muga SJ, Fischer SM. Prostaglandin E2 regulation of cyclooxygenase expression in keratinocytes is mediated via cyclic nucleotide-linked prostaglandin receptors. J Lipid Res. 2000;41:873–881. [PubMed] [Google Scholar]
- Moreno-Manzano V, Ishikawa Y, Lucio-Cazana J, Kitamura M. Suppression of apoptosis by all-trans-retinoic acid. Dual intervention in the c-Jun N-terminal kinase-AP-1 pathway. J Biol Chem. 1999;274:20251–20258. doi: 10.1074/jbc.274.29.20251. [DOI] [PubMed] [Google Scholar]
- Moreno-Manzano V, Sepúlveda JC, Jimenez J, Rodriguez M, Rodriguez D, Kitamura M, et al. Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. Br J Pharmacol. 2000;131:1673–1683. doi: 10.1038/sj.bjp.0703728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Mesangial cells orchestrate inflammation in the renal glomerulus. News Physiol Sci. 1994;9:271–276. [Google Scholar]
- Romero-Sandoval EA, Alique M, Moreno-Manzano V, Molina C, Lucio FJ, Herrero JF. The oral administration of retinoic acid enhances nociceptive withdrawal reflexes in rats with soft-tissue inflammation. Inflamm Res. 2004;53:297–303. doi: 10.1007/s00011-004-1261-5. [DOI] [PubMed] [Google Scholar]
- Sawano H, Haneda M, Sugimoto T, Inoki K, Koya D, Kikkawa R. 15-Deoxy-Delta12,14-prostaglandin J2 inhibits IL-1beta-induced cyclooxygenase-2 expression in mesangial cells. Kidney Int. 2002;61:1957–1967. doi: 10.1046/j.1523-1755.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer JJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cel Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Harendza S, Zahner G, Jocks T, Wenzel U, Wolf G, et al. Cyclooxygenase metabolites mediate glomerular monocyte chemoattractant protein-1 formation and monocyte recruitment in experimental glomerulonephritis. Kidney Int. 1999;55:430–441. doi: 10.1046/j.1523-1755.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL, Garavito M. Cyclooxygenases: structural, cellular and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Soler M, Camacho M, Sol R, Vila L. Mesangial cells release untransformed prostaglandin H2 as a major prostanoid. Kidney Int. 2001;59:1283–1289. doi: 10.1046/j.1523-1755.2001.0590041283.x. [DOI] [PubMed] [Google Scholar]
- Stahl RA, Thaiss F, Haberstroh U, Kahf S, Shaw A, Schoeppe W. Cyclooxygenase inhibition enhances rat interleukin 1 beta-induced growth of rat mesangial cells in culture. Am J Physiol. 1990;259:F419–F424. doi: 10.1152/ajprenal.1990.259.3.F419. [DOI] [PubMed] [Google Scholar]
- Stork PK, Schmitt JM. Crosstalk between cAMP and MAP kinase signalling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Thacher SM, Vasudevan J, Chandraratna RAS. Therapeutic applications for ligands of retinoid receptors. Curr Pharm Design. 2000;6:25–58. doi: 10.2174/1381612003401415. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Hishinuma T, Tayama R, Narahara K, Suzuki N, Tomioka Y, et al. The induction of prostaglandin E synthase and upregulation of cyclooxygenase-2 by 9-cis retinoic acid. Prostaglandins Other Lipid Mediat. 2004;74:61–74. doi: 10.1016/j.prostaglandins.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK-1) EMBO J. 2003;22:13–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Dechow C, Morath C, Lehrke I, Amann K, Waldherr RM, et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol. 2000;11:1479–1487. doi: 10.1681/ASN.V1181479. [DOI] [PubMed] [Google Scholar]
- Wardlaw SA, Zhang N, Belinsky SA. Transcriptional regulation of basal cyclooxygenase-2 expression in murine lung tumor-derived cell lines by CCAAT/enhancer-binding protein and activating transcription factor/cAMP response element-binding protein. Mol Pharmacol. 2002;62:326–333. doi: 10.1124/mol.62.2.326. [DOI] [PubMed] [Google Scholar]
- Xie W, Fletcher BS, Andersen RD, Herschman HR. V-Src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol. 1994;14:6531–6539. doi: 10.1128/mcb.14.10.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Konta T, Furusu A, Nakayama K, Lucio-Cazana FJ, Fine L, et al. Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids. Selective roles of nuclear receptors and contribution to the antiapoptotic effect. J Biol Chem. 2002;277:41693–41700. doi: 10.1074/jbc.M207095200. [DOI] [PubMed] [Google Scholar]
- Xu Q, Lucio J, Kitamura M, Ruan X, Fine L, Norman J. Retinoids in nephrology: promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- Zahner G, Wolf G, Ayoub M, Reinking R, Panzer U, Shankland SJ, et al. Cyclooxygenase-2 overexpression inhibits platelet-derived growth factor-induced mesangial cell proliferation through induction of the tumour suppressor gene p53 and the cyclin-dependent kinase inhibitors p21waf−1/cip−1 and p27kip−1. J Biol Chem. 2002;277:9763–9771. doi: 10.1074/jbc.M106307200. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Tao J, Zhu Q, Jia PM, Dou AX, Li X, et al. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18:285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.