Abstract
Background and purpose:
Desensitization of somatodendritic 5-HT1A receptors is involved in the mechanism of action of several antidepressants, but the rapidity of this effect and the amount of agonist stimulation needed are unclear. We evaluated the capacity of the high-efficacy 5-HT1A agonist, F13714 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone) and of the partial agonist, flesinoxan, to desensitize somatodendritic 5-HT1A receptors involved in the control of 5-HT release.
Experimental approach:
Intracerebral microdialysis in the hippocampus of freely moving rats was used to examine the acute and chronic effects of the two compounds (administered by osmotic pumps for 3, 7 or 14 days) on extracellular 5-HT levels, measured by HPLC with electrochemical detection.
Key results:
When given acutely, F13714, flesinoxan and the low-efficacy 5-HT1A agonist, buspirone, dose-dependently decreased extracellular 5-HT concentrations (ED50 values: 0.04, 0.77 and 5.6 mg kg−1, respectively). The selective 5-HT1A antagonist WAY100635 inhibited the effects of the three compounds. F13714 (2.5 mg kg−1 per day for 3, 7 or 14 days and 0.63 mg kg−1 for 7 days) significantly attenuated the inhibition of 5-HT release induced by buspirone (10 mg kg−1). In contrast, flesinoxan (10 mg kg−1 per day) failed to alter the response to buspirone at any of the treatment durations.
Conclusions and implications: Rat somatodendritic 5-HT1A receptors controlling hippocampal 5-HT release were rapidly desensitized by chronic activation with a high-efficacy 5-HT1A agonist, but not by chronic activation with a partial agonist. Thus, rapid 5-HT1A autoreceptor desensitization by high-efficacy agonists may accelerate the onset of the therapeutic effects of antidepressants.
Keywords: microdialysis, 5-HT1A receptors, extracellular 5-HT concentration, chronic administration, F13714, flesinoxan, osmotic pumps
Introduction
5-Hydroxytryptamine1A (5-HT1A) receptors are important targets for the treatment of mood disorders (Blier and Ward, 2003; Celada et al., 2004). For example, the partial 5-HT1A receptor agonist buspirone is widely prescribed for treating anxiety (see Fulton and Brogden, 1997) and drugs acting as agonists at 5-HT1A receptors have been shown to exhibit anxiolytic- and/or antidepressant-like activity in animal models (see De Vry, 1995).
5-HT1A receptors are located presynaptically on cell bodies in the raphe nuclei (somatodendritic receptors) and postsynaptically in 5-HT forebrain projecting areas. By activating somatodendritic receptors, 5-HT and 5-HT1A receptor agonists decrease the firing of 5-HT neurons in the raphe, and, consequently decrease 5-HT terminal release (see Barnes and Sharp, 1999). This decrease is thought to be responsible for the delay in onset of the therapeutic action, often by several weeks, of antidepressants, in particular selective 5-HT reuptake inhibitors (Blier and Ward, 2003). Indeed, a decrease in 5-HT release in 5-hydroxytryptaminergic projection areas prevents reuptake inhibitors from fully exerting their effect. In contrast, antagonism at 5-HT1A autoreceptors prevents the feed-back inhibition of 5-HT release. Therefore, blocking somatodendritic 5-HT1A receptors may reduce the delay in the onset of the effects of antidepressants. This has led to the administration of selective 5-HT reuptake inhibitors in combination with pindolol, a drug that is thought to act as an antagonist at somatodendritic 5-HT1A receptors (for review see Martinez et al., 2000). However, pindolol is not a selective 5-HT1A antagonist, but rather acts as a partial agonist at this site, and is a β-adrenoceptor blocking agent. In addition, the reported clinical effects of the combination are moderate and highly variable (Martinez et al., 2000). An alternative strategy for reducing the delay in an antidepressant's action is to use an efficacious 5-HT1A agonist that would rapidly desensitize somatodendritic 5-HT1A receptors and, at the same time, activate postsynaptic 5-HT1A receptors that mediate at least part of the therapeutic actions of antidepressants (Haddjeri et al., 1998; Blier and Ward, 2003). Indeed, desensitization of somatodendritic 5-HT1A receptors following repeated or chronic administration of 5-HT1A receptor agonists has been observed on the firing of raphe 5-HT neurons, second messenger activation, behavioural and neuroendocrine responses (for review see Hensler, 2003). In particular, changes have been observed in the electrophysiological response to 5-HT1A agonists after their repeated or sustained administration (Matheson et al., 1996; Haddjeri et al., 1999; Le Poul et al., 1999). Interestingly, the desensitization of 5-HT1A autoreceptors by antidepressants, but not by the low-efficacy 5-HT1A agonist gepirone, has been shown to lead to an increased tonic activation of postsynaptic 5-HT1A receptors in the hippocampus (Haddjeri et al., 1998).
However, the limited experimental data available on the effects of chronic or sustained administration of 5-HT1A agonists on 5-HT release are inconsistent. Using microdialysis to measure extracellular 5-HT in the striatum, Kreiss and Lucki (1992, 1997) showed desensitization of 5-HT1A autoreceptors after 7 or 14 days of treatment with the prototypic 5-HT1A receptor agonist, 8-OH-DPAT. The mechanism of this effect may be region-specific, as the authors failed to detect significant desensitization when measuring extracellular 5-HT in the hippocampus. In contrast, Sharp et al. (1993) found no tolerance to the effects of buspirone, ipsapirone or 8-OH-DPAT. Using subcutaneous osmotic pumps, and, at variance with the above-mentioned work, in freely moving animals, Casanovas et al. (1999) found a tolerance to the effects of the partial 5-HT1A agonist, alnespirone, but not to those of 8-OH-DPAT.
In this context, the present work sought to determine whether rapid desensitization of 5-HT1A receptors could be induced by treatment with a high-efficacy agonist compared with a partial agonist. F13714 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone), is a high-efficacy 5-HT1A agonist, as demonstrated in in vitro models of 5-HT1A receptor activation (Koek et al., 2001). Here, F13714 and flesinoxan, a 5-HT1A partial agonist (Newman-Tancredi et al., 2005), were administered chronically by means of subcutaneously (s.c.) implanted osmotic pumps for 3, 7 or 14 days. On day 4, 8 or 15, the effects of an acute dose of the low-efficacy 5-HT1A agonist buspirone on extracellular levels of 5-HT in the hippocampus were examined, using in vivo microdialysis.
Methods
Receptor-binding assays
F13714 was examined in vitro using membrane preparations from brain tissues or cell lines expressing recombinant receptors. Binding studies were performed as described previously in membranes from the brain area or cell line indicated, on the following receptor sites: 5-HT1A in rat hippocampus (Assié and Koek, 1999), h5-HT1A in Chinese hamster ovary (CHO) cells (Newman-Tancredi et al., 2005), h5-HT1B and h-5HT1D in Cos-7 cells (Pauwels et al., 1996) 5-HT2A in rat cortex, 5-HT2C in pig cortex (Koek et al., 1998), dopamine D1 in rat striatum (Kleven et al., 1997), α2-adrenoceptor in rat cortex (Hudson et al., 1992). Moreover, F13714 was tested in more than 40 other receptor, transporter and ion channel assays (Cerep, Celle l'Evescaut, France; data on file).
Animals
Male Sprague–Dawley rats (Ico: OFA SD (SPF Caw); Iffa Credo, France), (weight upon arrival: 160–180 g for the 14-day treatment, 200–220 g for the 7-day treatment and 240–260 g for the 3 day and acute treatments) were housed in groups (three rats per cage), under controlled conditions (12/12 h light/dark cycle: lights on 07 h 00 min; ambient temperature 21±1°C; humidity 55±5%), with rat food (AO4, UAR, France) and filtered (0.2 μm pore diameter) tap water freely available. At least 5 days were allowed for adaptation before the rats were used in the experiments. Experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, US National Research Council, 1996), and were approved by the institutional Ethical Review Committee.
Treatments
F13714 (0.63 and 2.5 mg kg−1 per day) and flesinoxan (10 mg kg−1 per day) were continuously administered using osmotic pumps (Alzet 2ML2 for 14-day treatment or Alzet 2ML1 for 3- and 7-day treatment). Doses were chosen to ensure the sustained presence of a 5-HT1A agonist at levels sufficient to activate somatodendritic receptors. Thus, 0.63 and 2.5 mg kg−1 F13714 per day correspond to 0.025 and 0.1 mg kg−1 per hour, doses that encompass the ED50 of the compound in acute experiments (0.04 mg kg−1, Table 1). For flesinoxan, the dose of 10 mg kg−1 per day corresponds to 0.42 mg kg−1 per hour, a dose just below its ED50 in acute experiments (0.77 mg kg−1, Table 1). Drugs were dissolved in distilled water and pumps were filled with the solutions. Pumps (filled with saline for control animals) were implanted s.c. under isoflurane anaesthesia. Rats were housed one per cage after surgery. One day before the microdialysis experiment, the pump was removed with the animal under chloral hydrate anaesthesia (immediately before the implantation of a guide cannula for the microdialysis probe; see below). All the pumps were checked for effectiveness at the end of the study by examining whether their contents had been released.
Table 1.
ED50 values of 5-HT1A agonists for decreasing extracellular concentration of 5-HT in rat hippocampus and their in vitro affinity (pKi) and efficacy (Emax; % effect relative to 10 μM 5-HT) at rat brain 5-HT1A receptors
| Compound | ED50 (mg kg−1) | pKia | Emax (%)a |
|---|---|---|---|
| F13714 | 0.04 | 10.01 | 75 |
| Flesinoxan | 0.77 | 8.87 | 55 |
| Buspirone | 5.6 | 7.87 | 20 |
5-HT1A; 5-hydroxytryptamine1a.
Data from: Koek et al. (2001), Newman-Tancredi et al. (2005) and unpublished observations.
Microdialysis procedure
Rats were anaesthetized with chloral hydrate (500 mg kg−1 intraperitoneally (i.p.)). A guide cannula (CMA 12, CMA/Microdialysis, Solna, Sweden) with a dummy probe was stereotaxically implanted into the ventral hippocampus, stereotaxic coordinates: rostral −4.8 mm, lateral +4.6 mm, ventral −4.6 mm, from bregma and skull surface according to Paxinos and Watson (1986). Two additional holes were drilled for skull screws and the guide was secured with dental cement.
Following surgery, animals were returned to their home cage. At the end of the day, each rat was placed in a microdialysis cage. On the following morning, the dummy probe was replaced by a microdialysis probe (3 mm length, 0.5 mm diameter, CMA 12, CMA/Microdialysis, Solna, Sweden). The probe was continuously perfused (1.1 μl min−1) with artificial cerebrospinal fluid containing 1 μM of the selective 5-HT reuptake inhibitor, citalopram. Starting approximately 2 h after probe implantation, samples were collected every 20 min. After four stable baseline samples (i.e.: s.e.m. <16%), (i) in acute experiments, saline or WAY100635 were injected s.c., followed 40 min later by i.p. injection of saline or the agonist, (ii) in chronic experiments, saline or buspirone were injected i.p. Samples were collected for a 140 min period after the last injection.
At the end of the experiment, animals were killed by injection of a lethal dose of pentobarbital (160 mg kg−1, i.p.) and the brain was removed, frozen and coronal sections were obtained with a cryomicrotome (Jung Frigocut 2800, Leica) to verify the placement of the probe.
Measures of 5-HT were performed by means of an online high performance liquid chromatography (HPLC) system with electrochemical detection as described previously (Assié et al., 2005). Concentrations of 5-HT were estimated by comparing peak areas from the microdialysis samples with those of external standards of known concentration of 5-HT. The limit of detection (three times baseline noise) was approximately 1 fmol/20 μl sample.
Data analysis
The perfusate levels of 5-HT are expressed as percent of the mean of the absolute quantity of transmitter collected in the four pre-injection control samples (basal level). Data were analysed using repeated measures analysis of variance (ANOVA) carried out with the Mixed procedure of SAS 8.2 software for PC (Littell et al., 2000). Percent measures taken after treatment administration were included in the statistical analysis of post-treatment effect (i.e. 20–140 min). Post hoc comparisons were made with the method of contrasts based on the Fisher's statistics (Myers and Well, 1995). For acute experiments the mean percent area under the curve (AUC) for the 140-min period after the administration of the agonist was used to calculate ED50 values estimated by linear interpolation between the two doses that decrease 5-HT levels with amounts bordering 50% (vehicle control as 0% and maximal effect of the compound as 100%).
Drugs
Buspirone hydrochloride was purchased from Sigma-RBI (Saint Quentin Fallavier, France), chloral hydrate from Acros (Geel, Belgium) and pentobarbital sodium from Ceva Santé Animale (Libourne, France). Citalopram was kindly donated by Lundbeck (Copenhagen, Denmark). Flesinoxan, WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide) dihydrochloride and F13714 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone) glycolate were synthesized at the Centre de Recherche Pierre Fabre. The compounds were dissolved in distilled water and the doses of compounds were expressed as the base. The volume of injection for acute administration was 10 ml kg−1. This volume of injection conforms to good practice in administration of substances (Diehl et al., 2001). All animal experiments at the Centre de Recherche Pierre Fabre follow these guidelines under recommendations of the institutional Ethical Review Committee.
Results
Receptor binding
F13714 exhibited high affinity for rat hippocampal 5-HT1A receptors and human 5-HT1A receptors expressed in CHO cells (pKi±s.e.m.: 10.01±0.05 and 10.40±0.09, respectively, n=3), consistent with previous findings in rat cortex (Koek et al., 2001). With the exception of sigma binding sites for which the IC50 was 77±29 nM, the affinity of F13714 for the other receptor, channel and enzyme binding sites examined (dopamine D1, hD3, hD4, hD5, adenosine A1, A2, α2, β1, β2 adrenoceptor, benzodiazepine, GABAA, GABAB, AMPA, kainate, NMDA, PCP, histamine H1, H2, H3, muscarinic, nicotinic, opiate, h5-HT1B, h5-HT1D, 5-HT3, 5-HT4, 5-HT6, 5-HT7 receptors, 5-HT, dopamine and noradrenaline uptake sites, calcium, potassium and sodium channels, acetylcholinesterase, MAO-A, MAO-B) was at least 1000-fold lower (less than 50% inhibition at 1 μM).
Effects of acute administration of the compounds on extracellular 5-HT levels
The mean basal extracellular concentration of 5-HT in the rat ventral hippocampus was 41.4±1.5 fmol 20 μl−1 (n=101) in the presence of 1 μM of the 5-HT reuptake inhibitor, citalopram.
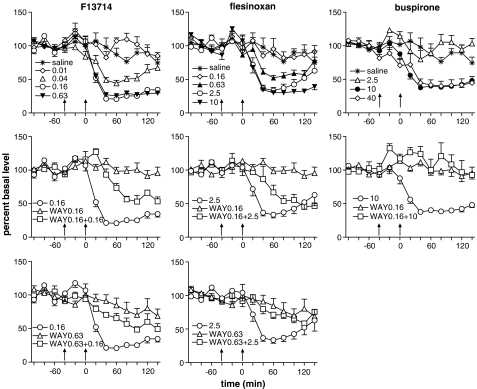
F13714 (0.01–0.63 mg kg−1, i.p.) dose dependently decreased 5-HT levels (Figure 1; Table 1) with an ED50 value of 0.04 mg kg−1. There was a significant effect of time (F6,232=13.3, P<0.0001) and treatment (F8,40=26.4, P<0.0001) and a significant interaction (F48,232=1.98, P=0.0005). Compared to controls, F13714 produced a significant decrease in extracellular 5-HT at 0.04, 0.16 and 0.63 mg kg−1 (P<0.0001). The selective 5-HT1A receptor antagonist, WAY100635 (0.16 and 0.63 mg kg−1, s.c.) administered 40 min before F13714 (0.16 mg kg−1) significantly attenuated its effects in a dose-dependent manner (P<0.0001).
Figure 1.
Effect of acute administration of the 5-HT1A agonists F13714, flesinoxan or buspirone alone (top panels) and together with WAY100635 (0.16 and 0.63 mg kg−1, s.c.; middle and bottom panels, respectively) on extracellular 5-HT levels in microdialysates from the ventral hippocampus of freely moving rats. Levels are expressed as the percentage of the mean absolute amount of 5-HT in the four samples collected before the injection of saline or WAY100635 (first arrow), the second arrow indicates injection of the 5-HT1A agonist or saline (n=5–7 animals per group).
Flesinoxan (0.16–10 mg kg−1, i.p.) dose dependently decreased 5-HT levels with an ED50 value of 0.77 mg kg−1. There was a significant effect of time (F6,232=13.1, P<0.0001) and treatment (F8,40=11.4, P<0.0001) and a significant interaction (F48,232=1.64, P=0.009). Compared to controls, flesinoxan produced a significant decrease in extracellular 5-HT at 0.63 (P=0.004), 2.5 and 10 mg kg−1 (P<0.0001). WAY100635 (0.16 and 0.63 mg kg−1, s.c.) administered 40 min before flesinoxan (2.5 mg kg−1) dose dependently attenuated its effects, this attenuation was significant at 0.63 mg kg−1 (P=0.002).
Buspirone (2.5–40 mg kg−1, i.p.) dose dependently decreased 5-HT levels with an ED50 value of 5.6 mg kg−1. There was a significant effect of time (F6,138=3.06, P=0.008) and treatment (F5,24=20.3, P<0.0001) but no significant interaction (F30,138=1.41, P=0.098). Compared to controls, buspirone produced a significant decrease in extracellular 5-HT at 10 and 40 mg kg−1 (P<0.0001). WAY100635 (0.16 mg kg−1, s.c.) administered 40 min before buspirone (10 mg kg−1) significantly and totally prevented its effects (P<0.0001).
Effects of chronic treatment on basal extracellular 5-HT levels
Three, 7 or 14 days of treatment with F13714 0.63 or 2.5 mg kg−1 per day or flesinoxan 10 mg kg−1 per day by means of s.c. implanted pumps, did not significantly alter the basal extracellular concentration of 5-HT (F3,22=2.5, P=0.09; F3,24=1.3, P=0.31; F3,24=0.63, P=0.60) (Table 2). Results indicated that the animals receiving 2.5 mg kg1 F13714 had a lower basal level after 3, 7 and 14 days of treatment. However, this was neither dose-, nor treatment duration-dependent. Moreover, the variations in baseline observed after chronic administration of the compounds are within the range of baseline values in naive animals.
Table 2.
Basal extracellular concentration of 5-HT in rat hippocampus after chronic treatment with 5-HT1A agonists
| Treatment (mg kg−1 per day) | 3 days fmol 20 μl−1 | 7 days fmol 20 μl−1 | 14 days fmol 20 μl−1 |
|---|---|---|---|
| Saline | 47.9±6.1 (10) | 47.7±4.7 (10) | 35.9±4.8 (10) |
| F13714 0.63 | 53.5±3.8 (5) | 48.7±5.0 (6) | 40.7±5.5 (6) |
| F13714 2.5 | 31.8±2.4 (5) | 37.3±3.9 (7) | 33.6±4.5 (6) |
| Flesinoxan 10 | 37.2±6.0 (6) | 41.7±5.0 (5) | 42.3±3.6 (6) |
5-HT1A; 5-hydroxytryptamine1a.
For each rat, basal extracellular concentration of 5-HT is the mean (fmol 20 μl−1)±s.e.m. of the four samples preceding buspirone injection. Data are mean±s.e.m. for the number of rats indicated in brackets.
Influence of a 3-day treatment with F13714 or flesinoxan on buspirone-induced decrease in extracellular 5-HT levels
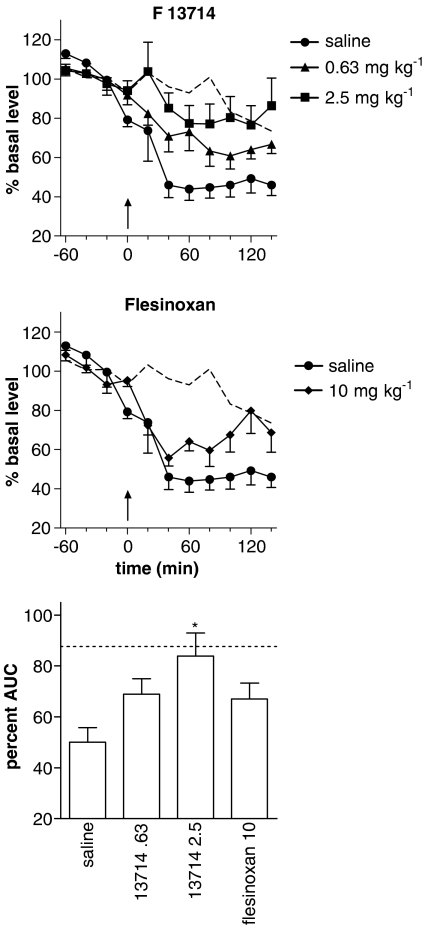
Following acute administration of buspirone (10 mg kg−1) after 3 days of treatment with F13714 (0.63 or 2.5 mg kg−1 per day) or flesinoxan (10 mg kg−1 per day) by means of s.c. implanted pumps, there was a significant effect of time (F6,122=4.6, P=0.0003) and treatment (F4,21=5.0, P=0.006) but no significant interaction (F24,122=1.1, P=0.36) on extracellular 5-HT. The effect of buspirone in animals treated with saline was significantly different from that in rats treated with F13714 2.5 mg kg−1 (P=0.003), but not from that administered F13714 0.63 mg kg−1 (P=0.08) or flesinoxan 10 mg kg−1 (P=0.09) (Figure 2).
Figure 2.
Effect of chronic administration with s.c. implanted osmotic pumps for 3 days of the 5-HT1A agonists: F13714 (0.63 or 2.5 mg kg−1 per day; top panel) or flesinoxan (10 mg kg−1 per day; middle panel) on the decrease in 5-HT levels induced by an acute dose of buspirone (10 mg kg−1 i.p.). Levels are expressed as the percentage of the mean absolute amount of 5-HT in the four samples collected before the injection of buspirone (arrow). Bottom panel: percent AUC for the 140 min after injection of buspirone (*P<0.05, compared to saline). The dotted line depicts mean 5-HT levels in animals pretreated with saline and receiving an acute injection of saline.
Influence of a 7-day treatment with F13714 or flesinoxan on buspirone-induced decrease in extracellular 5-HT levels
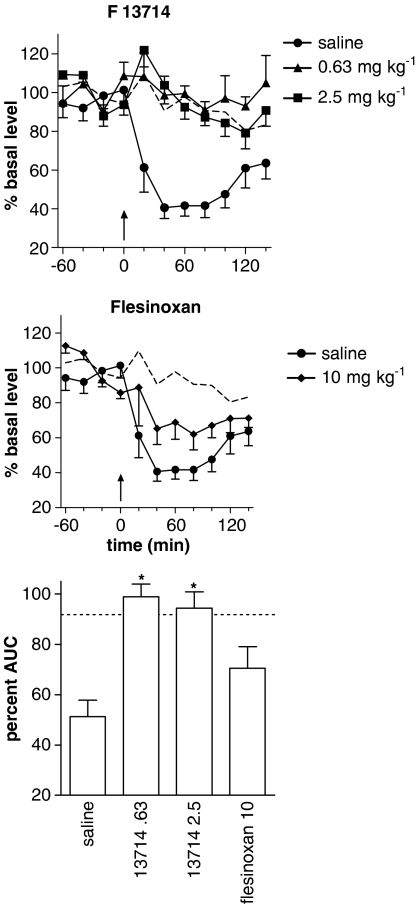
Following acute administration of buspirone (10 mg kg−1) after 7 days of treatment with F13714 (0.63 or 2.5 mg kg−1 per day) or flesinoxan (10 mg kg−1 per day), there was a significant effect of time (F6,136=5.3; P<0.0001) and treatment (F4,23=9.3; P=0.0001) but no significant interaction (F24,136=0.7, P=0.85) on extracellular 5-HT. The effect of buspirone in animals treated with saline was significantly different from that produced in rats treated with F13714 2.5 mg kg−1 (P<0.0001) and 0.63 mg kg−1 (P<0.0001) but not flesinoxan 10 mg kg−1 (P=0.06) (Figure 3).
Figure 3.
Effect of chronic administration with s.c. implanted osmotic pumps for 7 days of the 5-HT1A agonists: F13714 (0.63 or 2.5 mg kg−1 per day; top panel) or flesinoxan (10 mg kg−1 per day; middle panel) on the decrease in 5-HT levels induced by an acute dose of buspirone (10 mg kg−1 i.p.). Levels are expressed as the percentage of the mean absolute amount of 5-HT in the four samples collected before the injection of buspirone (arrow). Bottom panel: percent AUC for the 140 min after injection of buspirone (*P<0.05, compared to saline). The dotted line depicts mean 5-HT levels in animals pretreated with saline and receiving an acute injection of saline.
Influence of a 14-day treatment with F13714 or flesinoxan on buspirone-induced decrease in extracellular 5-HT levels
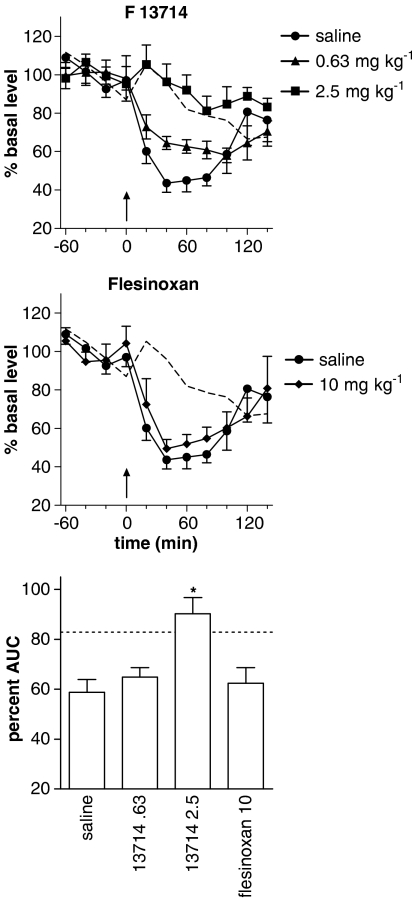
Following acute administration of buspirone (10 mg kg−1) after 14 days of treatment with F13714 (0.63 or 2.5 mg kg−1 per day) or flesinoxan (10 mg kg−1 per day), there was a significant effect of time (F6,135=3.3, P=0.005) and treatment (F4,23=5.8, P=0.002) but no significant interaction (F24,135=1.2, P=0.25) on extracellular 5-HT. The effect of buspirone in animals treated with saline was significantly different from that produced in rats treated with F13714 2.5 mg kg−1 (P=0.0007) but not 0.63 mg kg−1 (P=0.41) or flesinoxan 10 mg kg−1 (P=0.66) (Figure 4).
Figure 4.
Effect of chronic administration with s.c. implanted osmotic pumps for 14 days of the 5-HT1A agonists: F13714 (0.63 or 2.5 mg kg−1 per day; top panel) or flesinoxan (10 mg kg−1 per day; middle panel) on the decrease in 5-HT levels induced by an acute dose of buspirone (10 mg kg−1 i.p.). Levels are expressed as the percentage of the mean absolute amount of 5-HT in the four samples collected before the injection of buspirone (arrow). Bottom panel: percent AUC for the 140 min after injection of buspirone (*P<0.05, compared to saline). The dotted line depicts mean 5-HT levels in animals pretreated with saline and receiving an acute injection of saline.
The body weight of the animals after the different chronic treatments is shown on Table 3. Analyses of body weight, by one-way ANOVA, on the last day of chronic treatment for each treatment condition revealed no significant differences between the groups.
Table 3.
Body weight (in grams) at the end of the chronic treatment with 5-HT1A agonists
| Treatment (mg kg−1 per day) | 3 days | 7 days | 14 days |
|---|---|---|---|
| Saline | 311±9.4 (10) | 308±8.3 (10) | 319±5.7 (10) |
| F13714 0.63 | 308±9.5 (5) | 299±6.7 (6) | 303±8.1 (6) |
| F13714 2.5 | 305±7.2 (5) | 302±3.0 (7) | 313±6.3 (6) |
| Flesinoxan 10 | 301±10 (6) | 305±6.8 (5) | 320±8.3 (6) |
5-HT1A; 5-hydroxytryptamine1a.
Data are mean±s.e.m. for the number of rats indicated in brackets.
Discussion
The main finding of the present study is that F13714, a high-efficacy 5-HT1A agonist, desensitized somatodendritic 5-HT1A receptors within just 3 days of treatment. This desensitization was accentuated after 7 days, and was still present after 14 days of treatment. Treatment with flesinoxan, a partial agonist at 5-HT1A receptors, for similar durations failed to significantly desensitize the somatodendritic 5-HT1A receptors.
Influence of acute treatment with 5-HT1A agonists on extracellular 5-HT levels
All the 5-HT1A agonists with either high- or low-efficacy (such as buspirone), when administered acutely, decreased 5-HT release in terminal fields by acting on somatodendritic 5-HT1A receptors in the raphe, probably because of the high receptor reserve in this brain area (Meller et al., 1990; Cox et al., 1993). In the present study, acute administration of 5-HT1A agonists with different levels of efficacy (see Table 1) decreased 5-HT release down to 31% for F13714, 38% for flesinoxan and 43% for buspirone of control basal levels (=100%). These results are in agrement with those reported previously for flesinoxan (Bosker et al., 1996) and buspirone (Sharp et al., 1993; Matos et al., 1996; Assié and Koek, 2000). Consistent with their 5-HT1A agonist properties, the effects of all three compounds were antagonized by the selective 5-HT1A antagonist, WAY100635. Thus, the decrease in 5-HT extracellular levels induced by buspirone and flesinoxan was completely abolished by 0.16 mg kg−1 and 0.63 mg kg−1 WAY100635, respectively. Likewise, the higher dose of WAY100635 was more effective at antagonizing the effect of F13714. Nevertheless, complete blockade of the effects of F13714 was not achieved which suggests that this compound may act at additional targets. However, in view of the very high affinity, selectivity and efficacy of F13714 for 5-HT1A receptors, the most likely explanation is that higher doses of antagonist may be needed to occupy the receptors completely and prevent the response to F13714.
Influence of chronic treatment with 5-HT1A agonists on extracellular 5-HT levels
The present study focussed on the influence of chronic 5-HT1A agonist treatment on the response to acute administration of buspirone as an index of somatodendritic 5-HT1A receptor responsiveness. Indeed, sustained treatment with F13714 or flesinoxan induced no significant alteration of basal levels compared to saline control animals (see Results). The desensitization of somatodendritic 5-HT1A receptors, by alleviating the tonic inhibition produced by 5-HT, could have produced an increase in baseline; this was not observed here. This suggests that somatodendritic 5-HT1A receptors are not tonically activated (consistent with an absence of increase in extracellular 5-HT with the antagonist WAY100635 alone). Alternatively, the baseline decrease may have been so small as to be below the detection limit under present conditions.
The ability of buspirone to decrease extracellular 5-HT was abolished by sustained treatment with F13714, 2.5 mg kg−1 per day for 3, 7 or 14 days and 0.63 mg kg−1 per day for 7 days. In contrast, chronic treatment with flesinoxan, for any of the periods of treatment, failed to diminish the response to a buspirone. It is interesting that the present results differ from those of Haddjeri et al. (1999); they showed that chronic administration of flesinoxan desensitized the somatodendritic 5-HT1A receptors that modulate dorsal raphe firing. The reason for this may be related to differences in the methods used, although in both cases minipump administration was employed. In addition 5-HT release in the ventral hippocampus is regulated by 5-HT1A receptors originating both from the median and dorsal raphe (McQuade and Sharp, 1997); these receptors may vary in the manner in which they become desensitized. Alternatively, the regulation by flesinoxan of dorsal raphe firing may be mechanistically dissociated from 5-HT release, possibly owing to its interaction at other receptors such as dopamine D2 or 5-HT1B/1D. In fact, flesinoxan, unlike F13714, exhibits some modest affinity for these sites (Koek et al., 1998). Together, these results are consistent with the interpretation that 5-HT1A somatodendritic receptors desensitize more easily after sustained treatment with a high-efficacy agonist than with a compound exhibiting a more modest efficacy. It should be noted that the dose of flesinoxan, when expressed as mg kg−1 per hour, was lower than its ED50 determined in acute experiments. In contrast, the higher dose of F13714 was about two fold greater than its ED50 in acute studies. Therefore, it is possible that a higher dose of flesinoxan would desensitize the response to buspirone. However, because of limits imposed by its solubility, it was not possible to test a dose of flesinoxan higher than 10 mg kg−1 per day. Nevertheless, it is striking that none of the treatment durations with flesinoxan yielded a significant loss of sensitivity of 5-HT1A receptors. It is likely, therefore, that the lower efficacy of flesinoxan is responsible for its lack of effect on somatodendritic 5-HT1A responsiveness. In contrast, the desensitization observed with F13714 was significant within just 3 days of treatment and was maximal after 7 days. The reason why the treatment with 0.63 mg kg−1 F13714 produced significant desensitization after 7 but not 14 days is unclear. One possibility is that F13714 may provoke a sequence of overlapping regulatory effects (see following section). Thus a rapid desensitization owing to, for example, receptor internalization or post-translational modifications, may be followed by a slower effect at the level of receptor gene expression. Clarification of such issues would require studies of signal-transduction mechanisms but raise the possibility that additional levels of complexity exist in the modulation of somatodendritic 5-HT1A receptors.
Possible molecular mechanisms of somatodendritic 5-HT1A receptor desensitization
The mechanisms of desensitization of somatodendritic 5-HT1A receptors appear to be complex. Indeed, in addition to a possible downregulation of the receptor, regulatory changes distal to the receptor at the level of effector, regulation of G-protein expression, regulatory processes such as phosphorylation of G proteins or internalization of the receptor, may be involved.
Thus, at the receptor level, Beer et al. (1990) have shown a decrease in the number of 5-HT1A receptors in the raphe following a single administration of 8-OH-DPAT, possibly consecutive to receptor internalization (see Riad et al., 2001). This phenomenon may contribute to the rapid desensitization of 5-HT1A receptors observed with F13714. On the other hand, a downregulation of 5-HT1A receptors in the dorsal raphe has been reported after repeated administration of ipsapirone (Fanelli and McMonagle-Strucko, 1992) or alnespirone (Casanovas et al., 1999). However, these effects have not been consistently observed (see Schechter et al., 1990; Shiro et al., 1996; Le Poul et al., 1999; Hensler and Durgam, 2001). In the light of these data, sustained F13714 pretreatment may possibly downregulate somatodendritic 5-HT1A receptors, but this remains to be demonstrated. In contrast, the efficacy of flesinoxan may not have been sufficient to produce a downregulation of somatodendritic 5-HT1A receptors. It is also possible that chronic administration of F13714 induced an alteration of receptor G-protein coupling mediating the acute effect of buspirone. Indeed, after chronic administration of 8-OH-DPAT, 5-HT1A-stimulated [35S]-GTPγS binding was attenuated in the raphe and, to some extent, in the hippocampus, but not in other forebrain areas (Hensler and Durgam, 2001). Region-specific alterations of 5-HT1A receptor activated G-proteins (dorsal raphe and septum) have also been reported after chronic buspirone, although this compound has markedly lower agonist efficacy (Sim-Selley et al., 2000).
Desensitization of somatodendritic 5-HT1A receptors controlling 5-HT release
In microdialysis experiments, chronic treatments with 5-HT1A agonists have produced conflicting results. Twenty-four hours after a single administration of 8-OH-DPAT, no desensitization of the 5-HT1A receptors mediating the decrease in 5-HT release was observed in either the hippocampus or the striatum (Hjorth, 1991; Kreiss and Lucki, 1992). In contrast, after 7 days of treatment with 8-OH-DPAT, an acute dose of the compound did not decrease 5-HT release in the striatum (Kreiss and Lucki, 1997) indicating desensitization of the receptor, but a decrease was still observed in the hippocampus. After 2 weeks of treatment with 8-OH-DPAT administered by osmotic pumps, Casanovas et al. (1999) found no desensitization of the receptors mediating the decrease in 5-HT levels in the raphe and in the frontal cortex. In contrast, they reported that chronic alnespirone significantly attenuated the effects of an acute dose of the compound. However, when buspirone, a weak partial agonist at 5-HT1A receptors, was administered chronically for 14 days, 5 or 10 weeks, no desensitization of 5-HT1A autoreceptors was observed (Sharp et al., 1993; Söderpalm et al., 1993). Taken together, the above studies highlight two key findings. Firstly, in all the chronic 5-HT1A agonist treatment studies mentioned here, when desensitization occurred, at least 7–14 days of treatment were necessary to achieve it, with the exception of an internalization process that occurs within hours. In contrast, the results of the present study show that F13714 is potent at rapidly desensitizing somatodendritic 5-HT1A receptors. Indeed, it is possible that some desensitization could occur after acute injection of F13714, consistent with internalization/post-translational modification of 5-HT1A receptors. This would distinguish F13714 from other agonists, such as 8-OH-DPAT that fail to produce such rapid effects (see comments above). Secondly, the above studies also indicate a marked brain region-dependent variation in 5-HT1A receptor desensitization. Thus somatodendritic 5-HT1A receptors controlling 5-HT release in hippocampus may be differentially regulated compared with those controlling 5-HT release in the cortex or striatum. This issue would be interesting to explore further for F13714.
In conclusion, we have shown that rat somatodendritic 5-HT1A inhibitory receptors controlling hippocampal 5-HT release are rapidly desensitized by chronic activation with a high-efficacy 5-HT1A agonist but not by chronic activation with a partial agonist. Thus, rapid 5-HT1A autoreceptor desensitization by high-efficacy agonists may ameliorate the clinical effectiveness and accelerate the onset of therapeutic efficacy of antidepressant agents.
Conflicts of interest
The authors state no conflict of interest.
Acknowledgments
We thank Lundbeck for donation of citalopram, Nathalie Consul-Denjean, Christelle Benas, Jérôme Rouquet and Virginie Archimbeaud for technical assistance and Sophie Bréand for assistance with statistical analysis of data.
Abbreviations
- F13714
3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide dihydrochloride
References
- Assié M-B, Koek W. 8-OH-DPAT labels 5-HT1A receptors, 5-HT uptake sites as well as other serotonergic binding sites in the rat brain raphe area. Br J Pharmacol. 1999;126:255P. doi: 10.1038/sj.bjp.0703426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié M-B, Koek W. Estimation of apparent pA2 values for WAY 100635 at 5-HT1A receptors regulating 5-hydroxytryptamine release in anaesthetised rats. Eur J Pharmacol. 2000;409:173–177. doi: 10.1016/s0014-2999(00)00839-6. [DOI] [PubMed] [Google Scholar]
- Assié M-B, Ravailhe V, Faucillon V, Newman-Tancredi A. Contrasting contribution of 5-HT1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther. 2005;315:265–272. doi: 10.1124/jpet.105.087163. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beer M, Kennett GA, Curzon G. A single dose of 8-OH-DPAT reduces raphe binding of [3H]8-OH-DPAT and increases the effect of raphe stimulation on 5-HT metabolism. Eur J Pharmacol. 1990;178:179–187. doi: 10.1016/0014-2999(90)90473-j. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression. Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, De Winter TYCE, Klompmakers AA, Westenberg HGM. Flesinoxan dose-dependently reduces extracellular 5-hydroxytryptamine (5-HT) in rat median raphe and dorsal hippocampus through activation of 5-HT1A receptors. J Neurochemz. 1996;66:2546–2555. doi: 10.1046/j.1471-4159.1996.66062546.x. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Vilaró MT, Mengod G, Artigas F. Differential regulation of somatodendritic serotonin 5-HT1A receptors by 2-week treatments with the selective agonists alnespirone (S-20499) and 8-hydroxy-2-(di-n-propylamino)tetralin: microdialysis and autoradiographic studies in rat brain. J Neurochem. 1999;72:262–272. doi: 10.1046/j.1471-4159.1999.0720262.x. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Cox RF, Meller E, Waszczak BL. Electrophysiological evidence for a large receptor reserve for inhibition of dorsal raphe neuronal firing by 5-HT1A agonists. Synapse. 1993;14:297–304. doi: 10.1002/syn.890140407. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT1A receptor agonists: Recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Fanelli RJ, Mcmonagle-Strucko K. Alteration of 5-HT1A receptor binding sites following chronic treatment with ipsapirone measured by quantitative autoradiography. Synapse. 1992;12:75–81. doi: 10.1002/syn.890120109. [DOI] [PubMed] [Google Scholar]
- Fulton B, Brogden RN. Buspirone: an updated review of its clinical pharmacology and therapeutic applications. CNS Drugs. 1997;7:68–88. [Google Scholar]
- Haddjeri N, Blier P, De Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Ortemann C, De Montigny C, Blier P. Effect of sustained administration of the 5-HT1A receptor agonist flesinoxan on rat 5-HT neurotransmission. Eur Neuropsychopharmacol. 1999;9:427–440. doi: 10.1016/s0924-977x(99)00020-6. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Durgam H. Regulation of 5-HT1A receptor-stimulated [35S]-GTPγS binding as measured by quantitative autoradiography following chronic agonist administration. Br J Pharmacol. 2001;132:605–611. doi: 10.1038/sj.bjp.0703855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth S. Single-dose 8-OH-DPAT pretreatment does not induce tachyphylaxis to the 5-HT release-reducing effect of 5-HT1A autoreceptor agonists. Eur J Pharmacol. 1991;199:237–242. doi: 10.1016/0014-2999(91)90463-z. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Mallard NJ, Tyacke R, Nutt DJ. [3H]-RX821002: a highly selective ligand for the identification of α2-adrenoceptors in the rat brain. Mol Neuropharmacol. 1992;1:219–229. [Google Scholar]
- Kleven MS, Assié M-B, Koek W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT2A/2C antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther. 1997;282:747–759. [PubMed] [Google Scholar]
- Koek W, Patoiseau JF, Assié M-B, Cosi C, Kleven MS, Dupont-Passelaigue E, et al. F 11440, a potent, selective, high efficacy 5-HT1A receptor agonist with marked anxiolytic and antidepressant potential. J Pharmacol Exp Ther. 1998;287:266–283. [PubMed] [Google Scholar]
- Koek W, Vacher B, Cosi C, Assié M-B, Patoiseau JF, Pauwels PJ, et al. 5-HT1A receptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential. Eur J Pharmacol. 2001;420:103–112. doi: 10.1016/s0014-2999(01)01011-1. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Desensitization of 5-HT1A autoreceptors by chronic administration of 8-OH-DPAT. Neuropharmacology. 1992;31:1073–1076. doi: 10.1016/0028-3908(92)90110-b. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–116. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Fattaccini CM, Mocaër E, Hamon M, et al. Chronic alnespirone-induced desensitization of somatodendritic 5-HT1A autoreceptors in the rat dorsal raphe nucleus. Eur J Pharmacol. 1999;365:165–173. doi: 10.1016/s0014-2999(98)00886-3. [DOI] [PubMed] [Google Scholar]
- Littell RC, Miliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute Inc., SAS Campud Drive: Cary, NC, USA; 2000. [Google Scholar]
- Martinez D, Broft A, Laruelle M. Pindolol augmentation of antidepressant treatment: recent contributions from brain imaging studies. Biol Psychiatry. 2000;48:844–853. doi: 10.1016/s0006-3223(00)00993-8. [DOI] [PubMed] [Google Scholar]
- Matheson GK, Raess BU, Tunnicliff G. Effects of repeated doses of azapirones on rat brain 5-HT1A receptors and plasma corticosterone levels. Gen Pharmacol. 1996;27:355–361. doi: 10.1016/0306-3623(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Matos FF, Urban C, Yocca FD. Serotonin (5-HT) release in the dorsal raphe and ventral hippocampus: raphe control of somatodendritic and 5-HT release. J Neural Transm. 1996;103:173–190. doi: 10.1007/BF01292626. [DOI] [PubMed] [Google Scholar]
- Mcquade R, Sharp T. Functional mapping of dorsal and median raphe 5- hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Meller E, Goldstein M, Bohmaker K. Receptor reserve for 5-hydroxytryptamine1A-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytryptamine1A agonists. Mol Pharmacol. 1990;37:231–237. [PubMed] [Google Scholar]
- Myers JL, Well AD. Research Design and Statistical Analysis. Lawrence Elbraum Associates Inc.: Hillsdale, NJ, USA; 1995. [Google Scholar]
- Newman-Tancredi A, Assié M-B, Leduc N, Ormière A-M, Danty N, Cosi C. Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implication for treatment of schizophrenia. Int J Neuropsychopharmacol. 2005;11:1–16. doi: 10.1017/S1461145704005000. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Palmier C, Wurch T, Colpaert FC. Pharmacology of cloned human 5-HT1D receptor-mediated functional responses in stably transfected rat C6-glial cell lines: further evidence differentiating human 5-HT1D and 5-HT1B receptors. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:144–156. doi: 10.1007/BF00168751. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 1986Academic Press: New York; 2nd edn [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1A receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter LE, Bolanos FJ, Gozlan H, Lanfumey L, Haj-Dahmane S, Laporte AM, et al. Alterations of central serotoninergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: biochemical and electrophysiological studies. J Pharmacol Exp Ther. 1990;255:1335–1347. [PubMed] [Google Scholar]
- Sharp T, Mcquade R, Bramwell S, Hjorth S. Effect of acute and repeated administration of 5-HT1A receptor agonists on 5-HT release in rat brain in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:339–346. doi: 10.1007/BF00171331. [DOI] [PubMed] [Google Scholar]
- Shiro Y, Fujiwara Y HIKIJI M, Hamamura T, Shomori T, Kuroda S. Effect of chronic ipsapirone treatment on the density of 5-HT1A receptors and 5-HT1A receptor mRNA in discrete regions of the rat brain. Psychiatry Clin Neurosci. 1996;50:141–146. doi: 10.1111/j.1440-1819.1996.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Vogt LJ, Xiao RY, Childers SR, Selley DE. Region-specific changes in 5-HT1A receptor-activated G-proteins in rat brain following chronic buspirone. Eur J Pharmacol. 2000;389:147–153. doi: 10.1016/s0014-2999(99)00875-4. [DOI] [PubMed] [Google Scholar]
- Söderpalm B, Lundin B, Hjorth S. Sustained 5-hydroxytryptamine release-inhibitory and anxiolytic- like action of the partial 5-HT1A receptor agonist, buspirone, after prolonged chronic administration. Eur J Pharmacol. 1993;239:69–73. doi: 10.1016/0014-2999(93)90977-p. [DOI] [PubMed] [Google Scholar]