Abstract
Background and purpose:
Rapamycin is a widely used drug with antifungal, immunosuppressant and antiangiogenic effects. Herein, we studied whether immunosuppressive doses of rapamycin are capable of influencing endometriotic lesions.
Experimental approach:
We tested in vitro the potential of rapamycin to inhibit endothelial cell sprouting using the aortic ring assay and we further studied its effect on the expression of proliferating cell nuclear antigen (PCNA), apoptotic cell death-associated activated caspase-3 and vascular endothelial growth factor (VEGF) in cultured endometrial tissue fragments. In addition, we analyzed the drug in vivo after induction of endometriotic lesions by transplanting isolated endometrial fragments into the dorsal skinfold chamber of Syrian golden hamsters. Using intravital fluorescence microscopy, we repetitively analyzed angiogenesis, neovascularization and microcirculatory parameters over a time period of 14 days in rapamycin-treated animals and DMSO-treated controls.
Key results:
Administration of rapamycin significantly reduced the size of the endometriotic lesions. This was associated by inhibition of VEGF-mediated angiogenesis as indicated by a suppression of endothelial cell sprouting in vitro and a reduction of microvessel density in endometriotic lesions in vivo. Moreover, rapamycin directly inhibited cell proliferation within endometrial tissue, while manifestation of apoptotic cell death remained unaffected.
Conclusions and implications:
Our data indicate that administration of rapamycin may represent a novel therapeutic approach for an antiangiogenic treatment of endometriosis.
Keywords: rapamycin, sirolimus, angiogenesis, endometriosis, Syrian golden hamster, dorsal skinfold chamber, intravital fluorescence microscopy
Introduction
Rapamycin (sirolimus) is a bacterial macrolide, which is today widely used as an immunosuppressive drug to prevent rejection in organ transplantation. The drug acts by inhibiting the interleukin-2-stimulated T-cell division (Kahan, 2000; Yakupoglu and Kahan, 2003). After forming a complex with the FK binding protein (FKBP)-12, rapamycin blocks the mammalian target of rapamycin (mTOR), which is known to be a central controller of multiple mitogenic signaling pathways, regulating cell growth under physiological and pathological conditions (Wiederrecht et al., 1995; Schmelzle and Hall, 2000).
Interestingly, immunosuppressive doses of rapamycin have recently been shown to inhibit the development of tumors and tumor metastases, although the most widely recognized immunosuppressive drug cyclosporine promotes tumor growth (Guba et al., 2002). This specific effect of rapamycin has been suggested to be based on an antiangiogenic activity, as indicated by a reduced vascular endothelial growth factor (VEGF) production and a diminished VEGF-induced vascular endothelial cell stimulation (Guba et al., 2002). Because angiogenesis is a major prerequisite for the long-term survival and proliferation of tumors (Folkman, 2002), rapamycin has to be considered as a potential antiangiogenic agent in cancer therapy. This view is further supported by in vivo studies, which have demonstrated successful antiangiogenic treatment of pancreatic and CT-26 colon adenocarcinoma with rapamycin (Stephan et al., 2004; Guba et al., 2005a).
However, the development of new blood vessels represents a key step not only in tumor biology, but also in the pathogenesis of other angiogenic diseases including endometriosis. Endometriosis is one of the most frequent gynecological diseases nowadays and is defined by the presence and proliferation of functional endometrial glands and stroma outside the uterine cavity (Healy et al., 1998). In consideration of this fact, the aim of our study was to analyze in vitro and in vivo, whether rapamycin might also be capable of exerting antiangiogenic and antiproliferative effects in endometriotic lesions.
Methods
Animals
Eight- to 10-week-old female Syrian golden hamsters with a body weight of 60–90 g were used for the study. The animals were housed one per cage and had free access to tap water and standard pellet food (Altromin, Lage, Germany) throughout the experiment.
All experiments were conducted in accordance with the German legislation on protection of animals and the National Institute of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, WA, USA), and were approved by the local governmental animal care committee.
Preparation of the dorsal skinfold chamber
The dorsal skinfold chamber is a versatile tool to study the complex dynamic process of angiogensis in transplanted benign and malignant tissue (Menger et al., 2002). The chamber preparation contains one layer of striated muscle, subcutaneous tissue and skin and allows for intravital microscopic observation of the microcirculation in the awake animal over a period of time of up to 3 weeks. The chamber technique and its implantation procedure have been described previously in detail (Menger and Lehr, 1993). After the preparation, the animals were allowed to recover from anesthesia and surgery for at least 48 h.
Isolation and transplantation of endometrial fragments
For isolation of endometrial fragments, hamsters equipped with a dorsal skinfold chamber were anesthetized with pentobarbital sodium (50 mg kg−1 body weight intraperitoneally (i.p.)). After laparotomy, one uterus horn was aseptically removed and the laparotomy was closed with a two-layer 5–0 running suture. The explanted uterus horn was placed in a 30-mm-diameter Falcon plastic petri dish, containing 37°C warm Dulbecco's modified Eagle's medium (DMEM) (10% fetal calf serum, 1 U ml−1 penicillin, 0.1 mg ml−1 streptomycin; PAA Laboratories GmbH, Cölbe, Germany) and the fluorescent dye bisbenzimide H33342 (200 μg ml−1; Sigma, Taufkirchen, Germany). The specific fluorescence/background fluorescence ratio is high enough to precisely delineate the stained endometrial tissue from the non-stained surrounding host tissue after transplantation into the dorsal skinfold chamber. The isolated uterus horn was then opened longitudinally and the endometrium was dissected from the uterine muscle under a stereomicroscope. Subsequently, the endometrium was transferred into 37°C warm bisbenzimide H33342-free DMEM and microdissected into endometrial fragments of comparable size (∼0.5 mm2) (Laschke et al., 2005).
For autologous transplantation of endometrial fragments, the cover glass of the dorsal skinfold chamber was removed and four endometrial fragments were placed on the striated muscle within each chamber (Laschke et al., 2006). Before transplantation, all animals were hormonally synchronized, that is, in the same stage of the 4-day estrus cycle in the hamster, in order to exclude differences in the angiogenic process between the individual animals owing to hormonal variations. Endometrial fragments were transplanted on estrus. Synchronization was performed by administration of two subcutaneous injections of 55 μg kg−1 body weight estradiol (Sigma), given 24 h apart, followed by one injection of 7.5 mg kg−1 body weight of progesterone (Sigma), given 20 h after the last estradiol injection (Gross, 1977).
Intravital fluorescence microscopy
For intravital fluorescence microscopy, the animals were immobilized in a Plexiglas tube and the dorsal skinfold preparation was attached to the microscopic stage. After intravenous (i.v.) injection of 0.1 ml 5% fluorescein isothiocyanate (FITC)-labeled dextran 150 000, intravital fluorescence microscopy was performed using a modified Leitz Orthoplan microscope with a 100 W mercury lamp attached to a Ploemo-Pak illuminator with blue, green and ultraviolet filter blocks (Leitz, Wetzlar, Germany) for epi-illumination (Vollmar et al., 2001). The microscopic images were recorded by a charge-coupled device video camera (CF8/1 FMC; Kappa GmbH, Gleichen, Germany) and transferred to a video system for offline evaluation.
Microcirculatory analysis
Quantitative offline analysis of the videotapes was performed by means of a computer-assisted image analysis system (CapImage; Zeintl, Heidelberg, Germany) and included the determination of the size of the transplanted endometrial fragments (mm2), the size of the blood perfused microvascular networks (given in percent of the size of the grafts), the microvessel density, that is, the length of red blood cell (RBC)-perfused microvessels per area of observation (cm cm−2), the diameters of the microvessels (μm) and the centerline RBC velocity VRBC (μm s−1). Volumetric blood flow (VQ) of individual microvessels was calculated from VRBC and diameter (d) for each microvessel as VQ=π × (d/2)2 × VRBC/K (pl s−1), where K (=1.3) represents the Baker/Wayland factor (Baker and Wayland, 1974), considering the parabolic velocity profile of blood in microvessels.
Experimental protocol
A total of 28 endometrial fragments were transplanted into the dorsal skinfold chambers of seven female hamsters treated daily with rapamycin (1.5 mg kg−1 body weight/day i.p. in 150 μl dimethylsulfoxide (DMSO); Wyeth Pharma GmbH, Münster, Germany). A total of 20 endometrial fragments were transplanted into the dorsal skinfold chambers of five DMSO-treated (150 μl/day i.p.) control hamsters.
Intravital fluorescence microscopic analysis of the microcirculation was performed on days 0 (day of transplantation), 2, 4, 7, 10 and 14 after transplantation of endometrial fragments. Microvessel density was measured within three regions of interest per graft and observation time point. Microvascular diameters and microhemodynamic parameters were determined by analyzing 10 microvessels per region of interest. In all microvessels selected, both vessel diameter and VRBC were determined for subsequent calculation of VQ. At the end of the in vivo experiments the animals were killed with an overdose of pentobarbital, and the dorsal skinfold chamber preparations were processed for histological and immunohistochemical studies.
Aortic ring assay
To further evaluate the effect of rapamycin on endothelial cell sprouting, an in vitro angiogenesis assay was used. Aortic rings of Syrian hamsters were embedded in 200 μl matrigel (basement matrix; BD Biosciences, Heidelberg, Germany) in 48-well tissue culture grade plates and allowed to polymerize for 30 min at 37°C and 5% CO2. The wells were then overlaid with 800 μl of DMEM supplemented with rapamycin (30 μM dissolved in DMSO) or DMSO (1:1000), respectively. The rings were maintained at 37°C and 5% CO2 for 7 days with medium change every 2 days. All assays were carried out in quadruplicate in a total of three animals. Vascular sprouting from each ring was examined by transillumination phase-contrast microscopy. Images were recorded by means of an optronics engineering device (TEC-470; SI GmbH, Gilching, Germany) and transferred to a video system for off-line evaluation (CapImage). Quantitative analyses were performed at day 7 and included the determination of the area (mm2) and the maximal length (μm) of the outer aortic endothelial cell sprouting.
Western blot analysis
To investigate the effect of rapamycin on protein expression of proliferating cell nuclear antigen (PCNA), activated caspase-3 and VEGF within endometrial tissue, both uterus horns were isolated in three synchronized Syrian hamsters. Subsequently, endometrial fragments of the left uterus horn were cultured in DMEM supplemented with rapamycin (30 μM dissolved in DMSO). Endometrial fragments of the corresponding right uterus horn, which were cultured in DMEM with DMSO (1:1000) alone, served as controls. After 48 h, the tissue was removed and stored in liquid nitrogen for Western blot analysis.
For extraction of the whole-protein fraction, frozen tissue samples were homogenized in lysis buffer, incubated for 30 min on ice and centrifuged for 30 min at 16 000 g (4°C). The supernatant was saved as whole-protein fraction. Protein concentrations were determined using the Lowry assay with bovine serum albumin (BSA) as standard. Sixty microgram protein/lane were separated discontinuously on 10% sodium dodecylsulfate polyacrylamide gels and transferred to a polyvinyldifluoride membrane (BioRad, München, Germany). After blockade of nonspecific binding sites, membranes were incubated for 2 h with a mouse-monoclonal anti-PCNA antibody (1:500; DAKO Cytomation, Hamburg, Germany), a rabbit-polyclonal anti-cleaved caspase-3 antibody (1:800; Cell Signaling, Frankfurt, Germany) and a mouse-monoclonal anti-VEGF antibody (1:100; DAKO Cytomation) followed by the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000; Amersham Biosciences, Freiburg, Germany). Protein expression was visualized using luminal-enhanced chemiluminescence and exposure of membranes to blue light-sensitive autoradiography film (Hyperfilm ECL, Amersham Biosciences). Signals were densitometrically assessed (Geldoc, Quantity one software, BioRad) and normalized to β-actin signals (mouse anti-β-actin antibody, 1:5000; Sigma) to correct unequal loading.
Histology and immunohistochemistry
For light microscopy, formalin-fixed specimens were embedded in paraffin at day 14 after transplantation. Four-micrometer-thick sections were cut and stained with hematoxylin and eosin. For immunohistochemical detection of proliferating cells within the endometrial grafts PCNA staining was performed by a mouse monoclonal anti-PCNA antibody as primary antibody (1:50; Dako Cytomation). This was followed by a HRP-conjugated goat anti-mouse antibody (1:200; Amersham Biosciences). 3,3′ diaminobenzidine was used as chromogen. Counterstaining was performed with hemalaun.
Statistics
Data were first analyzed for normal distribution and equal variance. Differences between groups were then calculated by the unpaired Student's t-test. To test for time effects within each experimental group, analysis of variance for repeated measures was applied. This was followed by a post hoc paired comparison, including correction of the α-error according to Bonferroni probabilities for repeated measurements (SigmaStat; Jandel Corporation, San Rafael, CA, USA). All data are given as means±s.e.m. Statistical significance was accepted for a value of P<0.05.
Results
Intravital fluorescence microscopy
During the isolation procedure, endometrial tissue was microdissected in fragments of comparable size in order to exclude differences in growth factor content and release after transplantation into the dorsal skinfold chamber. Accordingly, endometrial fragments of rapamycin-treated and DMSO-treated control animals revealed an initial graft size of 0.52±0.07 and 0.49±0.02 mm2, respectively. During the following days, endometrial grafts in rapamycin-treated animals showed a regression over time, resulting in a significantly (P<0.05) reduced size of the grafts at day 14 with only ∼52% of that measured at day 0 (Figure 1). In contrast, the size of endometrial grafts in control animals remained constant during the entire 14-day observation period (Figure 1).
Figure 1.
(a, b) Intravital fluorescence microscopy of endometrial grafts (borders marked by broken line) at day 14 after autologous transplantation into the dorsal skinfold chamber of a control (a) and a rapamycin-treated (b) Syrian golden hamster. Blue-light epi-illumination with contrast enhancement by 5% FITC-labeled dextran 150 000 i.v. Scale bars: 60 μm. (c) Size of endometrial grafts (given in % of the initial size of the grafts) at day 14 after autologous transplantation into the dorsal skinfold chambers of control (open bars) and rapamycin-treated (closed bars) Syrian golden hamsters, as assessed by intravital fluorescence microscopy and computer-assisted image analysis. Means±s.e.m. *P<0.05 vs control.
The development of new blood vessels could be observed in both experimental groups. Angiogenesis already occurred at day 2 after transplantation and was characterized by the formation of capillary buds and sprouts ingrowing into the endometrial grafts. Subsequently, these sprouts interconnected with each other and finally formed a new microvascular network. Owing to their glomerulum-like angioarchitecture the newly formed microvessels could be clearly distinguished from the host striated muscle capillaries of the dorsal skinfold chamber preparation, which display a typical parallel arrangement (Figure 2a). In both groups, endometrial fragments were already revascularized at day 7 after transplantation (Figure 2c). However, during the following days, rapamycin treatment resulted in a slight regression of the grafts' microvascular networks with a significantly (P<0.05) reduced vascularized area of 87±4% at day 14 when compared to control animals (Figure 2b and c). Moreover, microvascular networks of endometrial fragments in rapamycin-treated hamsters revealed a significantly (P<0.05) reduced microvessel density of ∼170 cm cm−2 when compared to those of control animals exhibiting a microvessel density of ∼300 cm cm−2 (Figure 2b and d).
Figure 2.
(a, b) Intravital fluorescence microscopy of the newly formed microvascular networks of endometrial grafts (borders marked by arrows) at day 14 after autologous transplantation into the dorsal skinfold chamber of a control (a) and a rapamycin-treated (b) Syrian golden hamster. Note that both endometrial grafts are characterized by a newly formed microvascular network of glomerulum-like structure. However, the rapamycin-treated graft shows a smaller vascularized area and a lower microvessel density, but larger microvessel diameters (b). Blue-light epi-illumination with contrast enhancement by 5% FITC-labeled dextran 150 000 i.v. Scale bars: 100 μm. (c, d) Vascularized area (%) and microvessel density (cm cm−2) of endometrial grafts after autologous transplantation into the dorsal skinfold chambers of control (open circles) and rapamycin-treated (closed circles) Syrian golden hamsters, as assessed by intravital fluorescence microscopy and computer-assisted image analysis. Means±s.e.m. *P<0.05 vs control animals at corresponding time points; aP<0.05 vs day 0 within each individual group; bP<0.05 vs days 0 and 2 within each individual group; cP<0.05 vs days 0, 2 and 4 within each individual group.
In both control and rapamycin-treated hamsters, newly formed blood vessels presented with initial diameters of ∼16–17 μm at day 2 after transplantation (Table 1). During the following days, quantitative analysis of the vessel diameters revealed a progressive decrease in control animals to ∼11 μm at day 14. In contrast, vessel diameters in rapamycin-treated hamsters remained significantly (P<0.05) elevated between days 7 and 14 (Table 1).
Table 1.
Microvessel diameter (μm), centerline red blood cell velocity (μm s−1) and volumetric blood flow (pl s−1) of endometrial grafts after autologous transplantation into the dorsal skinfold chambers of hormonally synchronized Syrian golden hamsters, which were treated daily with rapamycin or vehicle (control)
| Day 2 | Day 4 | Day 7 | Day 10 | Day 14 | |
|---|---|---|---|---|---|
| Microvessel diameter (μm) | |||||
| Rapamycin | 16.8±1.0 | 15.3±0.4a | 14.1±0.4a* | 13.9±0.8a* | 13.1±0.4a* |
| Control | 16.2±1.0 | 14.8±0.7 | 11.7±0.3b | 11.5±0.4b | 11.3±0.2b |
| Centerline red blood cell velocity (μm s−1) | |||||
| Rapamycin | 46.4±18.9 | 95.3±10.1 | 172.8±16.4b | 238.7±22.8c | 215.6±28.3b |
| Control | 17.7±2.4 | 117.9±28.2a | 190.8±14.4a | 179.0±19.9a | 181.7±19.0a |
| Volumetric blood flow (pl s−1) | |||||
| Rapamycin | 6.5±2.0 | 12.8±1.5a | 21.3±2.2b | 27.9±5.5b | 22.2±1.9b* |
| Control | 3.0±0.6 | 14.7±3.0a | 15.6±1.3a | 14.7±2.6a | 14.2±1.6a |
Means±s.e.m.
P<0.05 vs day 2 within each individual group;
P<0.05 vs day 2 and 4 within each individual group;
P<0.05 vs day 2, 4 and 7 within each individual group;
P<0.05 vs control animals at corresponding time points.
Centerline RBC velocity showed an increase from ∼20–50 μm s−1 at day 2 to 180–220 μm s−1 at the end of the observation period without any significant differences between the experimental groups studied (Table 1). Calculated values of VQ ranged from 3 pl s−1 at day 2 to 14 pl s−1 at day 14 in control animals (Table 1). In comparison, rapamycin treatment resulted in an increased (P<0.05) VQ of ∼22 pl s−1 at day 14 after transplantation.
Aortic ring assay
Incubation of matrigel-embedded aortic rings with rapamycin could confirm our intravital microscopic finding that rapamycin effectively suppresses angiogenesis as demonstrated by a significantly reduced area and maximal length of the outer aortic endothelial cell sprouting when compared to DMSO-incubated control rings (Figure 3).
Figure 3.
(a, b) Representative images of aortic rings with vascular sprouting upon 7 days of incubation in DMEM supplemented with DMSO (a) or rapamycin (b), respectively. (c, d) Area (mm2) and maximal length (μm) of the outer aortic endothelial cell sprouting at day 7 after incubation of aortic rings, which were incubated in DMEM supplemented with DMSO (control, open bars) or rapamycin (closed bars), as assessed by transillumination phase-contrast microscopy and computer-assisted image analysis. Means±s.e.m. *P<0.05 vs control.
Western blot analysis
In endometrial fragments, which were cultured in DMEM supplemented with rapamycin, expression of PCNA and VEGF was significantly reduced when compared to controls (Figure 4). Of interest, the expression of activated caspase-3 did not show significant differences between the two experimental groups (Figure 4).
Figure 4.
Western blots (a) and Western blot analysis (b) of PCNA (37 kDa), activated caspase-3 (18–20 kDa) and VEGF (24 kDa) protein expression (optical density (OD)*mm2) of endometrial fragments, which were incubated for 48 h in DMEM supplemented with DMSO (control, open bars) or rapamycin (closed bars), respectively. Means±s.e.m. *P<0.05 vs control.
Histology
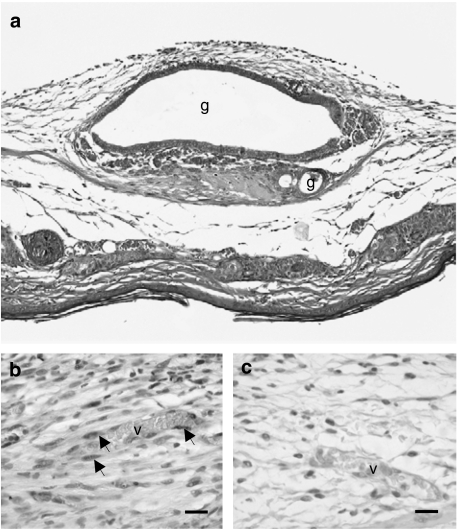
Transplantation of endometrial fragments into the dorsal skinfold chambers of both rapamycin-treated and control animals resulted in the establishment of endometriotic lesions, which were histologically characterized by cyst-like dilated endometrial glands surrounded by a vascularized endometrial stroma (Figure 5a). Within these lesions, newly formed blood vessels exhibited a normal vessel histomorphology with an intact endothelium. Moreover, these vessels were regularly perfused with RBCs without any signs of fibrin deposition or microthrombus formation.
Figure 5.
(a) Hematoxylin-eosin stained cross section of an endometrial graft at day 14 after transplantation onto the striated muscle tissue within the dorsal skinfold chamber of a rapamycin-treated Syrian golden hamster. The graft is characterized by cyst-like dilated endometrial glands (g) with an intact glandular epithelium surrounded by a vascularized endometrial stroma. Scale bar: 200 μm. (b, c) Immunohistochemical detection of PCNA in an endometriotic lesion of a control (b) and a rapamycin-treated (c) Syrian golden hamster at day 14 after transplantation of endometrial fragments into the dorsal skinfold chamber. Note that many stromal and endothelial cells (arrows) lining the walls of newly formed blood vessels (v) proliferate in the endometriotic lesion of the control animal (b), whereas the lesion of the rapamycin-treated hamster exhibits a significantly lower number of proliferating cells (c). Scale bars: 30 μm.
Immunohistochemical detection of PCNA revealed proliferation of many stromal and endothelial cells lining the vessel walls in endometriotic lesions of control animals (Figure 5b). In contrast, endometriotic lesions of rapamycin-treated hamsters exhibited only a few proliferating cells, which were mainly localized within the endometrial stroma (Figure 5c).
Discussion
Presently, rapamycin is a widely used drug, which has been shown to have antifungal, immunosuppressant and antitumor activities (Guba et al., 2002, 2005b; Yakupoglu and Kahan, 2003). Herein, we now suggest a new therapeutic application for rapamycin as an antiangiogenic agent in the treatment of endometriosis. As we could demonstrate in vivo, administration of rapamycin resulted in the regression of endometriotic lesions with a significant reduction of the size of the lesions in a short time period of 14 days. This effect was most probably owing to the inhibition of angiogenesis as indicated by a reduced microvessel density and vascularized area of the newly formed microvascular networks within these endometriotic lesions.
Generally, two potential mechanisms have been described previously, which could explain the antivascular effect of rapamycin. On the one hand, rapamycin suppresses the production of VEGF (Guba et al., 2002), one of the most potent angiogenic growth factors. This finding suggests a direct inhibitory effect on the angiogenic process by blockade of growth factor stimulated vascular endothelial cell activity. Correspondingly, we could demonstrate in the present study the downregulation of VEGF expression within endometrial tissue by rapamycin. Moreover, rapamycin treatment resulted in an effective inhibition of endothelial cell sprouting in the aortic ring assay. Additionally, rapamycin has been shown to disrupt newly formed microvascular networks in tumors owing to the formation of vessel thrombosis, which was mediated by the upregulation of tissue factor expression in tumor endothelial cells (Guba et al., 2005b). However, this observation seems to be a tumor specific effect, because in our study newly formed blood vessels in rapamycin-treated endometriotic lesions were regularly perfused with RBCs and exhibited a normal vessel histomorphology without any signs of fibrin deposition or microthrombus formation. Thus, we conclude that rapamycin primarily impairs the vascularization of endometriotic lesions by inhibition of VEGF-mediated angiogenesis. In this context, the excessive production and release of various angiogenic growth factors by endometriotic lesions (Taylor et al., 2002) might explain our observation that rapamycin treatment was not sufficient to prevent angiogenesis completely.
Interestingly, our intravital microscopic investigations showed that diameters of newly formed blood vessels progressively decreased in endometriotic lesions of control animals, whereas vessel diameters in rapamycin-treated hamsters remained significantly elevated between days 7 and 14. This observation might be a sign of impaired blood vessel maturation in rapamycin-treated animals, because the diameter of newly formed blood vessels normally decreases over time owing to the stabilization of the vessel wall by pericyte recruitment (Risau, 1997; Carmeliet, 2000; Laschke et al., 2002). However, a lack of pericyte stabilization would result in vessel wall irregularities with an extensive variability of the vessel diameters (Hellström et al., 2001; Laschke et al., 2006), which was not the case in our study. Therefore, we suggest that according to the study of Ryschich et al. (2004) the observed vessel dilatation rather represents an adaptive mechanism, which might help rapamycin-treated endometriotic lesions to expand their overall endothelial surface for the diffusion of oxygen and nutrition in order to partly compensate for the insufficient vascularization. Correspondingly, rapamycin treatment resulted in an increased VQ at day 14, when compared to control animals. This increase in VQ within the remaining microvessels during antiangiogenic therapy may not be interpreted as specific for endometriotic lesions, because similar effects have also been shown during antiangiogenic therapy of tumors (Vajkoczy et al., 1999). The remaining microvessels may in fact be considered as ‘fare-through' channels, which despite high-flow conditions cannot adequately serve for the nutritional supply of the tissue.
Beside its antiangiogenic activity, we could also demonstrate a direct inhibitory effect of rapamycin on cell proliferation within isolated endometrial tissue as indicated by the downregulation of PCNA expression. Immunohistochemical examinations of endometriotic lesions further revealed that especially the proliferating activity of stromal and endothelial cells was reduced. This is a surprising finding, because former studies reported that rapamycin has antiproliferative activity against normal nonimmunologic cells (Wiederrecht et al., 1995) and colon-38 tumor cells (Eng et al., 1984) only at extremely high doses of the drug (100–400 mg kg−1/day i.p.). However, for our in vivo investigations we used a relatively low immunosuppressive dose of rapamycin of 1.5 mg kg−1/day i.p., which has previously been shown to have an optimal antiangiogenic effect against CT-26 colon adenocarcinoma tumors (Guba et al., 2005a). Moreover, our Western blot analyses revealed that apoptotic cell death remained unaffected in rapamycin-treated endometrial tissue when compared to controls. In contrast, Stephan et al. (2004) found an almost 2-fold induction of pancreatic tumor cell apoptosis by rapamycin. Thus, it cannot be excluded that the effects of rapamycin on cell proliferation and programmed cell death are extremely dependent on the type of tissue under investigation. This might offer the opportunity to apply rapamycin as a selective drug not only for the anticancer therapy of different tumor types, but also for the treatment of endometriotic lesions.
In summary, we could demonstrate in the present study that immunosuppressive doses of rapamycin result in regression of endometriotic lesions owing to inhibition of VEGF-mediated angiogenesis and cell proliferation. Thus, administration of rapamycin might represent a novel therapeutic approach for antiangiogenic treatment of endometriosis.
Acknowledgments
We are grateful for the technical assistance of Janine Becker (Institute for Clinical and Experimental Surgery, Homburg/Saar). This work was supported by a grant of the Wilhelm Sander-Foundation (Nr. 2002.008.01) and the research program of the Medical Faculty of the University of Saarland (HOMFOR A/2004/09).
Abbreviations
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulfoxide
- FITC
fluorescein isothiocyanate
- FKBP-12
FK binding protein-12
- mTOR
mammalian target of rapamycin
- PCNA
proliferating cell nuclear antigen
- VEGF
vascular endothelial growth factor
- RBC
red blood cell
Conflict of interest
The authors state no conflict of interest.
References
- Baker M, Wayland H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc Res. 1974;7:131–143. doi: 10.1016/0026-2862(74)90043-0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot. 1984;37:1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Gross GH. A technique for sustained synchronization of hamster estrous cycles by hormonal means. Horm Behav. 1977;9:23–31. doi: 10.1016/0018-506x(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Guba M, Koehl GE, Neppl E, Doenecke A, Steinbauer M, Schlitt HJ, et al. Dosing of rapamycin is critical to achieve an optimal antiangiogenic effect against cancer. Transplant Int. 2005a;18:89–94. doi: 10.1111/j.1432-2277.2004.00026.x. [DOI] [PubMed] [Google Scholar]
- Guba M, Yezhelyev M, Eichhorn ME, Schmid G, Ischenko I, Papyan A, et al. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005b;105:4463–4469. doi: 10.1182/blood-2004-09-3540. [DOI] [PubMed] [Google Scholar]
- Healy DL, Rogers PAW, Hii L, Wingfield M. Angiogenesis: a new theory for endometriosis. Hum Reprod Update. 1998;4:736–740. doi: 10.1093/humupd/4.5.736. [DOI] [PubMed] [Google Scholar]
- Hellström M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Elitzsch A, Vollmar B, Menger MD. In vivo analysis of angiogenesis in endometriosis-like lesions by intravital fluorescence microscopy. Fertil Steril. 2005;84 (Suppl 2):1199–1209. doi: 10.1016/j.fertnstert.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21:262–268. doi: 10.1093/humrep/dei308. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD, Vollmar B. Ovariectomy improves neovascularization and microcirculation of freely transplanted ovarian follicles. J Endocrinol. 2002;172:535–544. doi: 10.1677/joe.0.1720535. [DOI] [PubMed] [Google Scholar]
- Menger MD, Laschke MW, Vollmar B. Viewing the microcirculation through the window: some twenty years experience with the hamster dorsal skinfold chamber. Eur Surg Res. 2002;34:83–91. doi: 10.1159/000048893. [DOI] [PubMed] [Google Scholar]
- Menger MD, Lehr HA. Scope and perspectives of intravital microscopy – bridge over from in vitro to in vivo. Immunol Today. 1993;14:519–522. doi: 10.1016/0167-5699(93)90179-O. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Ryschich E, Schmidt E, Maksan SM, Klar E, Schmidt J. Expansion of endothelial surface by an increase of vessel diameter during tumor angiogenesis in experimental and hepatocellular and pancreatic cancer. World J Gastroenterol. 2004;10:3171–3174. doi: 10.3748/wjg.v10.i21.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Stephan S, Datta K, Wang E, Li J, Brekken RA, Parangi S, et al. Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin Cancer Res. 2004;10:6993–7000. doi: 10.1158/1078-0432.CCR-04-0808. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100. doi: 10.1111/j.1749-6632.2002.tb02769.x. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Menger MD, Vollmar B, Schilling L, Schmiedek P, Hirth KP, et al. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia. 1999;1:31–41. doi: 10.1038/sj.neo.7900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar B, Laschke MW, Rohan R, Koenig J, Menger MD. In vivo imaging of physiological angiogenesis from immature to preovulatory ovarian follicles. Am J Pathol. 2001;159:1661–1670. doi: 10.1016/S0002-9440(10)63013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht GJ, Sabers CJ, Brunn GJ, Martin MM, Dumont FJ, Abraham RT. Mechanism of action of rapamycin: new insights into the regulation of G1-phase progression in eukaryotic cells. Prog Cell Cycle Res. 1995;1:53–71. doi: 10.1007/978-1-4615-1809-9_5. [DOI] [PubMed] [Google Scholar]
- Yakupoglu YK, Kahan BD. Sirolimus: a current perspective. Exp Clin Transplant. 2003;1:8–18. [PubMed] [Google Scholar]