Abstract
Background and purpose:
Antagonists of Ca2+ channels reduce contraction of intestinal smooth muscle but also affect vascular smooth muscle. We have therefore examined the effects of AJG049, a newly synthesized antagonist for regulation of gut motility, on voltage-dependent L-type Ca2+ channels, in vascular and intestinal smooth muscle, comparing AJG049 with two other Ca2+ channel antagonists, verapamil and diltiazem.
Experimental approach:
Affinities of AJG049 for various types of voltage-dependent Ca2+ channels were examined by binding studies. Effects of AJG049 on voltage-dependent inward Ca2+ (or Ba2+) currents (ICa or IBa) in dispersed smooth muscle cells from guinea-pig ileum, colon and mesenteric artery were measured using conventional whole-cell configurations.
Key results:
In binding studies, AJG049 showed a high affinity for the diltiazem-binding site of L-type Ca2+ channels. In whole-cell configuration, AJG049 suppressed ICa in ileal myocytes, with concentration-, voltage-and use-dependencies. AJG049 shifted the steady-state inactivation curve of ICa to the left. The order of potency to inhibit ICa in ileal myocytes was AJG049>verapamil>diltiazem. AJG049 also suppressed IBa in guinea-pig mesenteric arterial myocytes, showing concentration- and voltage-dependencies and the potency order for this action was also AJG049>verapamil>diltiazem. For the relative ratio of Ki values between ileal and mesenteric arterial myocytes, the order was AJG049>diltiazem≫verapamil.
Conclusions and implications:
These results show that AJG049 inhibits L-type Ca2+ channels mainly through the diltiazem-binding site(s). From our results, AJG049 showed a little selectivity for these Ca2+ channels in intestinal smooth muscle.
Keywords: AJG049, Ca2+ channel antagonists, intestinal smooth muscle, irritable bowel syndrome, L-type Ca2+ channels, tissue selectivity
Introduction
It is well known that voltage-dependent Ca2+ channels in gastrointestinal smooth muscle play a significant role in regulating not only membrane excitability but also bowel motility as one of the major Ca2+ entry pathways (reviewed by Bolton et al., 1999). As several Ca2+ channel antagonists, such as nifedipine, diltiazem and verapamil, inhibit voltage-dependent Ca2+ channels in intestinal smooth muscle (Terada et al., 1987; Duridanova et al., 1993), Ca2+ channel antagonists reduce contraction of smooth muscle. Thus, it is generally thought that these Ca2+ channel antagonists may be useful to moderate the abnormal motility in patients with irritable bowel syndrome (IBS) (verapamil, Byrne, 1987; diltiazem, Johnson et al., 1988). However, these drugs also produce dose-dependent cardiovascular effects (e.g. hypotensive action) over similar dose ranges as those required to inhibit hypermotility in gastrointestinal smooth muscle (reviewed by Traube and McCallum, 1984). Much effort is currently being spent to synthesize selective Ca2+ channel antagonists for the treatment of hypermotility disorders of gastrointestinal tract smooth muscle.
AJG049 (R-(+)-5,11-dihydro-5-[1-(4-methoxyphenethyl)-2-pyrrolidinylmethyl] dibenzo [b,e] [1,4] oxazepine hydrochloride) is a newly developed Ca2+ channel antagonist. The purpose of the present study was to investigate whether or not AJG049 may possess a tissue selectivity for intestinal smooth muscle in comparison with that for vascular smooth muscle, observing the effects of AJG049 on voltage-dependent Ca2+ (or Ba2+) currents (ICa or IBa) in enzymatically dispersed single guinea-pig intestinal (ileum and colon) and vascular (mesenteric artery) smooth muscle cells using conventional whole-cell recording techniques. We have further compared the potency of the drug with those of other types of well-known Ca2+ channel antagonists (verapamil and diltiazem) in whole-cell recordings. Moreover, radioligand receptor-binding assays were performed to identify high-affinity binding sites for AJG049 in several voltage-dependent Ca2+ channels.
Methods
Radioligand binding assay for various voltage-dependent Ca2+ channels in rat cerebral cortex
Male Sprague–Dawley rats were killed by decapitation, and the brain was quickly removed. The cerebral cortex was dissected from the rest of the brain on a Petri dish over ice. The tissue was then added to separate centrifugation tubes containing 19 volumes of 50 mM Tris-HCl (pH 7.4), and the tissue homogenized for 10 s repeated three times. The cerebral cortical homogenate was washed twice (48 000 g for 10 min) and re-suspended in 50 mM Tris-HCl (pH 7.4). Radioligand binding studies with d-cis-[3H]diltiazem, (+)-[3H]PN200-110, [3H]desmethoxyverapamil ([3H]D888) and [125I]ω-conotoxin GVIA were carried out at 25°C in 50 mM Tris-HCl (pH 7.4) as described previously (Lee et al., 1983), with minor modifications. Radioligand concentration, protein concentration and incubation time of competition binding experiments were 2.61 nM, 5 mg tissue eq ml−1 and 120 min for d-cis-[3H]diltiazem; 0.49 nM, 10 mg tissue eq ml−1 and 90 min for (+)-[3H]PN200-110; 0.49 nM, 5 mg tissue eq ml−1 and 30 min for [3H]D888; and 0.008 nM, 10 mg tissue eq ml−1 and 30 min for [125I]ω-conotoxin GVIA, respectively. Nonspecific binding was measured in the presence of 100 μM diltiazem for d-cis-[3H]diltiazem binding, 100 μM nifedipine for (+)-[3H]PN200-110 binding, 100 μM D600 for [3H]D888 binding and 10 μM ω-conotoxin GVIA for [125I]ω-conotoxin GVIA binding. At the end of the incubation period, samples were diluted with 3 ml of ice-cold washing buffer (50 mM Tris-HCl, pH 7.4) and immediately filtered through GF/C filters (glass microfibre filter type C, Whatman International Ltd, Maidstone, UK), which had been presoaked in 0.5% polyethyleneimine. Filters were washed three times with 3 ml of ice-cold buffer. A Brandel cell harvester was used for the filtration procedure. Radioactivity on the filter was measured by liquid scintillation counting or gamma counting.
Cell dispersion
Male guinea-pigs weighing 300–400 g were killed by cervical dislocation and exsanguination. A segment of ileum, colon or mesenteric artery (first branch; 1.0–1.5 cm) was excised and rinsed thoroughly in Ca2+-free physiological salt solution (PSS) (mM): Na+ 140, K+ 5, Mg2+ 1.2, glucose 5, Cl− 151.4, HEPES 10, titrated to pH 7.40 with Tris base. A segment of the tissue was incubated at 35°C in Ca2+-free PSS containing collagenase (intestinal muscle, 1.3 mg ml−1, type I, Sigma-Aldrich Japan KK, Tokyo, Japan; mesenteric artery, 1 mg ml−1, Wako Pure Chem. Ind. Ltd, Osaka, Japan) for 20–25 min. Thereafter, the digested segment of the thin longitudinal layer was carefully peeled off with fine forceps and scissors under a binocular microscope and stored in 0.5 mM Ca2+-containing PSS containing 1 mg ml−1 fatty acid-free bovine serum albumin at 4–6°C. The cells were dispersed for each experiment by mincing the segments into pieces and then triturating them with a blunt-tipped Pasteur pipette. The spindle-shaped dispersed cells were stored at 4°C and normally used within 4 h for experiments.
Recording procedure
Patch-clamp experiments were performed at room temperature (21–23°C) as described previously (Hamill et al., 1981; Teramoto et al., 2001). Briefly, patch electrodes were prepared with an electrode puller (P-97, Sutter Instrument Company, Novato, CA, USA) and a heat polisher (MF-83, Narishige Sci. Inst. Lab., Tokyo, Japan). A high-resistance seal (>10 GΩ) was obtained by application of a negative pressure to the patch pipette (2–5 MΩ). Voltage-clamp recordings were obtained with the conventional whole-cell configuration. Generation of voltage pulses was performed using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA, USA). The sampled current data were filtered at 10 kHz and stored together with potential records on videotape using a video recorder (PCM-501ES, SONY, Tokyo, Japan) for subsequent off-line analysis. Junction potentials between bath and pipette solutions were measured using a 3 M KCl reference electrode and were <2 mV, so that correction for these potentials was not made. Capacitance was kept to a minimum by maintaining the test solution in the bath as low as possible. At the beginning of each experiment, the series resistance was compensated, adjusting the transient cancellation control to provide the best cancellation.
Solutions
The following solutions were used for whole-cell recording. PSS (mM): Na+ 140, K+ 5, Mg2+ 1.2, Ca2+ 2, glucose 5, Cl− 151.4, HEPES 10 and was titrated to pH 7.35–7.4 with Tris base. The pipette solution contained (mM) Cs+ 130, tetraethylammonium (TEA+) 10, Mg2+ 4, Cl− 148, creatine phosphate 5, EGTA 5, ATP 5, HEPES 10/Tris (pH 7.35–7.4). For recording IBa in whole-cell configuration, Ba2+ 10 mM bath solution contained (mM) Ba2+ 10, TEA+ 135, Cl− 155, glucose 10, HEPES 10/Tris (pH 7.35–7.40).
Data analysis and statistics
Whole-cell current data were low-pass filtered at 500 Hz by an eight-pole Bessel filter, sampled at 1 ms intervals and analysed on a computer (Macintosh PowerPC 7100/80AV, Apple Computer Japan, Tokyo, Japan) by commercial software ‘MacLab 3.4.2' (ADInstruments Pty Ltd, Castle Hill, Australia). The amplitudes of whole-cell currents were measured at peak height. Measurements of the amplitude of ICa were corrected for background membrane current by subtraction of currents obtained when voltage command pulses were applied to the cells in the presence of extracellular Cd2+ (50 μM).
The peak amplitude of the Ca2+ (or Ba2+) current elicited by a step pulse to 0 mV (+10 mV for the Ba2+ current) from the holding potential just before application of a drug was normalized as one. The curves were drawn by fitting the following equation using the least-squares method.
Relative amplitude of ICa=1/{1+([D]/Ki}nH, where Ki, [D] and nH are the inhibitory dissociation constant, concentration of drug (nM) and Hill's coefficient, respectively.
The dissociation constant for drug binding to the channel to the inactivated state could be estimated from the shift of the voltage-dependent inactivation curve and the concentration response curve obtained at the resting state by use of the following equation (Bean et al., 1983; Teramoto, 1993),
where ΔVhalf is the amplitude of the shift of the voltage-dependent inactivation curve, k is a slope factor for the inactivation curve and [D] is the concentration of drug applied. Kinact and Krest are dissociation constants of AJG049 for the inactivated and the resting states of voltage-dependent Ca2+ channels, respectively.
Statistical analyses were performed with Student's t-test for paired values. Changes were considered significant at P<0.01. Data are expressed as mean with s.e.m.
Materials
The following chemicals were used: AJG049 (Pharmaceutical Research Laboratories of Ajinomoto Co., Tokyo, Japan), prepared daily as a 10 mM stock solution in ethanol and diluted just before use. The final concentration of ethanol was less than 0.1%. Other drugs and chemicals were obtained from Sigma Chemicals (Sigma Chemical KK, Tokyo, Japan).
Results
Affinities of AJG049 for voltage-dependent Ca2+ channels in rat cerebral cortex
In order to investigate the affinities of AJG049 in several voltage-dependent Ca2+ channels, the affinities of AJG049 for two kinds of voltage-dependent Ca2+ channels (L-type and N-type) were evaluated by use of radioligand receptor binding assays in rat cerebral cortex. The values were determined for AJG049 by nonlinear regression analysis of their competition curves (see Methods). AJG049 possessed the highest affinity for the diltiazem-binding site of L-type Ca2+ channels (IC50=79 nM), compared with other binding sites of L-type Ca2+ channels (verapamil site, IC50=245 nM; dihydropyridine site, IC50≫10 μM) and N-type Ca2+ channels (IC50≫10 μM).
The effects of AJG049 on ICa in guinea-pig intestinal smooth muscle
To investigate the effects of AJG049 on ICa, conventional whole-cell recordings were performed in guinea-pig ileal myocytes. When the recording pipette was filled with a Cs+-TEA+ solution containing 5 mM EGTA and the bath solution was physiological saline solution (PSS) containing 2 mM Ca2+, application of a depolarizing step to −40 mV from a holding potential of −60 mV produced an inward Ca2+ current (Figure 1a). This current increased slightly with time after establishing the whole-cell configuration, reaching a steady level approximately 4 min after rupture of the membrane patch (n=10). This peak value was then maintained at least for 15 min, whereas test depolarization pulses (1 s duration) were applied at 30 s intervals (the peak amplitude of ICa at 15 min being 99±2% (n=25) of the value determined 4 min after the establishment of whole-cell recording). Consequently, all experiments were performed within a 15 min period.
Figure 1.
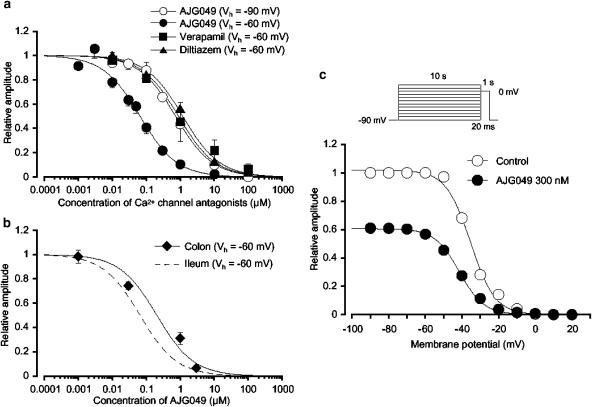
Effects of AJG049 on ICa at a holding membrane potential of −60 mV in guinea-pig ileal myocytes. Whole-cell recording, pipette solution Cs+-TEA+ solution containing 5 mM EGTA and bath solution PSS. (a) Original current traces before (control) and after the application of 10 nM AJG049. (b) Current–voltage relationships obtained in the absence (control) or presence of AJG049 (10 nM, 1 and 10 μM). The current amplitude was measured as the peak amplitude of ICa in each condition. The lines were drawn by eye. (c) Relationship between the test potential and relative value of ICa inhibited by 10 nM AJG049, expressed as a fraction of the peak amplitude of ICa evoked by depolarizing pulses of various amplitudes in the absence of AJG049. Each symbol indicates the mean of five observations with ±s.e.m. shown by vertical lines. Some of the s.e.m. bars are smaller than the size of the symbol. The line was drawn by eye.
As shown in Figure 1a, AJG049 (10 nM) inhibited the peak amplitude of ICa evoked by depolarizing pulses (1 s duration) from a holding potential of −60 mV at levels more positive than −20 mV. Figure 1b shows the current–voltage relationships in the absence and presence of AJG049 (10 nM, 1 and 10 μM), and the inhibition showed concentration-dependent behaviour. AJG049 (10 μM) suppressed the peak amplitude of ICa (Figure 1b). In Figure 1c, the AJG049 (10 nM)-induced inhibition showed a voltage dependency (n=5). The peak amplitude of ICa was also suppressed by 10 μM nifedipine, a selective L-type Ca2+ channel blocker (data not shown). Figure 2a shows the relationships between the relative peak amplitude of ICa and concentrations of AJG049 at two different holding potentials (−60 and −90 mV). ICa was evoked by a depolarizing pulse to 0 mV applied every 30 s. AJG049 inhibited the peak amplitude of ICa in a concentration-dependent manner (−60 mV, Ki=66.5 nM; −90 mV, Ki=739.1 nM). To compare the inhibitory potency of AJG049 (Ki=66.5 nM) with that of other Ca2+ channel antagonists at −60 mV, verapamil and diltiazem were applied (Figure 2a). These Ca2+ channel antagonists reduced the peak amplitude of ICa in a concentration-dependent manner as reported previously. In guinea-pig colonic myocytes, AJG049 also suppressed the peak amplitude of ICa in a concentration-dependent manner, showing less potency than in guinea-pig ileal myocytes (Figure 2b). The voltage dependency was investigated before and after the application of 300 nM AJG049 using the experimental protocol shown in Figure 2c (conditioning pulse duration, 10 s; holding membrane potential, −90 mV). In the absence of AJG049 (control), inactivation of ICa occurred with depolarizing pulses positive to −50 mV in guinea-pig ileal myocytes. After the application of 300 nM AJG049 (approximately 5 min later), the voltage-dependent inactivation curve in the same cells was significantly shifted to the left by 6±1 mV (n=4; Figure 2c).
Figure 2.
Effects of AJG049 on ICa in guinea-pig ileal myocytes. Whole-cell recording, pipette solution Cs+-TEA+ solution containing 5 mM EGTA and bath solution PSS. (a) Concentration–response curves for AJG049 at two holding potentials (−60 and −90 mV) and other Ca2+ channel antagonists (verapamil and diltiazem) at −60 mV on ICa in guinea-pig ileal myocytes. The peak amplitude of ICa elicited by a step pulse to 0 mV from the holding potential just before the application of AJG049 was normalized as one. The curves were fitted and the following values were used: AJG049, −60 mV, Ki=66.5 nM, nH=0.8; −90 mV, Ki=739.1 nM, nH=0.8; verapamil, Ki=901.6 nM, nH=0.8; diltiazem, Ki=1.2 μM, nH=0.8. Each symbol indicates the mean of 4–8 observations with s.e.m. shown by vertical lines. Some of s.e.m. bars are smaller than the size of the symbol. (b) Concentration–response curve for AJG049 on ICa in guinea-pig colonic myocytes at a holding potential of −60 mV (Ki=207.8 nM, nH=0.8). The curve with the broken line was obtained from (a) regarding the inhibitory effects of AJG049 on ICa in guinea-pig ileal myocytes at −60 mV. Each symbol indicates the mean of 4–8 observations with ±s.e.m. shown by vertical lines. Some of s.e.m. bars are smaller than the size of the symbol. (c) At −90 mV, conditioning pulses of various amplitudes were applied (up to +30 mV, 10 s duration) before the application of the test pulse (to 0 mV, 1 s duration). An interval of 20 ms was allowed between these two pulses to allow estimation of possible contamination by capacitive current. The peak amplitude of ICa evoked by each test pulse was measured before and after the application of 300 nM AJG049. The lines were drawn by fitting the data to the following equation in the least-squares method, I=Imax/{1+exp[(V–Vhalf)/k]} where I is the peak amplitude of ICa observed at various amplitudes of the conditioning pulse and Imax is the response to the application of a conditioning pulse from −90 mV, V is the amplitude of the conditioning pulse and Vhalf the amplitude of the conditioning pulse that evokes ICa of half-maximum amplitude and k is the slope factor. The curves in the absence or presence of AJG049 were fitted using the following values: control, Imax=1.0, Vhalf=−35.2 mV and k=6.7, n=4; AJG049, Imax=0.6, Vhalf=−41.3 mV and k=6.7, n=4.
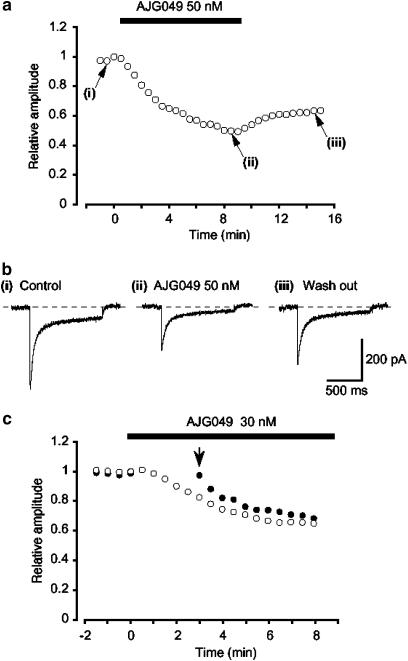
Figure 3a shows the time course of the effects of AJG049 (50 nM) on ICa evoked by a depolarizing pulse to 0 mV from −60 mV. The depolarizing pulses were applied every 30 s. When the peak amplitude of ICa just before application of AJG049 (control) was taken as one, AJG049 reduced the peak amplitude of ICa to approximately 60% (n=5; Figure 3b). On removal of AJG049, the peak amplitude gradually recovered, but not to the control level (Figure 3a and b). As shown in Figure 3c, when a depolarizing pulse was applied after an interval of 3 min in the presence of 30 nM AJG049, the peak amplitude of ICa was smaller than that observed before the application of AJG049 but consistently larger than that recorded at 4 min with repetitive application of the depolarizing pulses (single pulse, 1.0±0.01, n=5 versus repetitive pulse 0.8±0.05, n=5; P<0.01).
Figure 3.
The time course of changes in the peak amplitude of ICa evoked by repetitive depolarizing pulses to 0 mV from a holding potential of −60 mV before and after the application of AJG049. The abscissa shows the time (min). Time 0 indicates the time at which AJG049 was added to the bath. The ordinate shows the relative size of the peak amplitude of ICa when the peak amplitude of ICa in the absence of AJG049 (i.e. control) was normalized as one. (a) The typical time course of application of 50 nM AJG049 on the peak amplitude of ICa. (b) Original current traces before (control (i)) and after application of 50 nM AJG049 (ii), as indicated in (a). (iii) Original current trace indicates a current trace following removal of AJG049. (c) No pulses were applied for the initial 3 min after application of 30 nM AJG049. The peak amplitude of ICa just before the application of AJG049 was normalized as one (control).
Voltage-dependent inhibitory effects of AJG049 on IBas in guinea-pig mesenteric arterial myocytes
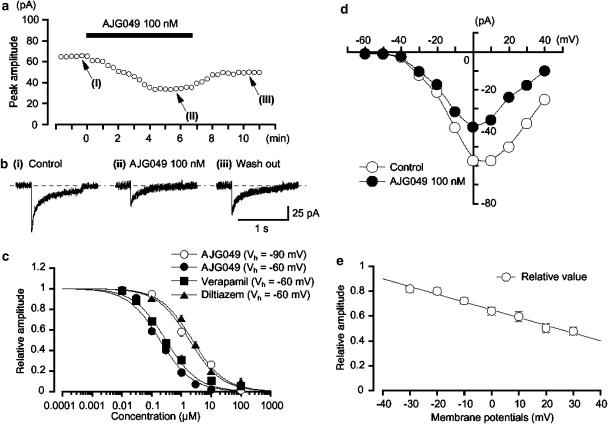
To further study whether or not AJG049 possesses tissue selectivity between gut and vascular smooth muscles, the effects of AJG049 on IBa were investigated in guinea-pig mesenteric arterial myocytes, as it has previously been reported that the peak amplitude of nifedipine-sensitive ICa in guinea-pig mesenteric arterial myocytes was too small (less than 20 pA at +10 mV) to estimate precisely (Ohya et al., 1997). Thus, in the present experiments, Ba2+ (10 mM) was used as a charge carrier in the bath solution in order to enhance the amplitude of the inward currents for analysis and to isolate IBa by inhibiting other Ca2+-activated mechanisms (such as Ca2+-activated K+ currents and Ca2+-activated Cl− currents). The recording pipette was filled with a Cs+-TEA+ solution containing 5 mM EGTA. Figure 4a shows the time course of the effects of AJG049 (100 nM) on IBa (20 s interval). Application of 100 nM AJG049 gradually reduced the peak amplitude of the inward current, nearly halving it within a few minutes (n=10; Figure 4b). On removal of AJG049, the peak amplitude of IBa gradually recovered, but did not return to control level (0.71±0.04, n=5; Figure 4b). Subsequent application of nifedipine (10 μM) completely suppressed the currents (data not shown). Figure 4c shows the relationship between the relative peak amplitude of IBa evoked by a depolarizing pulse to +10 mV from two different holding membrane potentials (−60 and −90 mV) applied every 20 s and concentrations of AJG049. AJG049 inhibited the peak amplitude of IBa in a concentration- and voltage-dependent manner (−60 mV, Ki, 190.3 nM; −90 mV, Ki=1.9 μM). Similarly, other Ca2+ channel antagonists (verapamil and diltiazem) reduced the peak amplitude of IBa in a concentration-dependent manner at a holding potential of −60 mV (Ki, verapamil, 300.8 nM; diltiazem, 2.4 μM; Figure 4c). The rank order of potency of the Ki values for ICa (or IBa) was AJG049>verapamil>diltiazem in guinea-pig ileal and mesenteric arterial myocytes, although the Ki values were different between two tissues. As shown in Figure 4d, AJG049 inhibited the peak amplitude of IBa evoked by depolarizing pulses (1 s duration) from a holding potential of −60 mV at levels more positive than −30 mV. Figure 4d shows the current–voltage relationships in the absence and presence of 100 nM AJG049. In Figure 4e, the AJG049 (100 nM)-induced inhibition showed a voltage dependency (n=5). The value of the tissue selectivity was calculated as a relative ratio of the Ki values between guinea-pig ileal (Figure 2a) and mesenteric arterial myocytes (Figure 4c), indicating that the rank order of a relative ratio was AJG049 (2.9)>diltiazem (2.0)≫verapamil (0.3).
Figure 4.
Effects of AJG049 on IBa in dispersed smooth muscle cells from guinea-pig mesenteric artery. Whole-cell recording, pipette solution Cs+-TEA+ solution containing 5 mM EGTA and bath solution 10 mM Ba2+ containing 135 mM TEA+. (a) The time course of the effects of application of AJG049 (100 nM) on the peak amplitude of IBa evoked by repetitive depolarizing pulses to +10 mV from a holding potential of −60 mV. Time 0 indicates the time when AJG049 was applied to the bath. (b) Original current traces before (control (i)) and after the application of 100 nM AJG049 (ii), as indicated in (a). (iii) Original current trace indicates a current trace following the removal of AJG049. (c) Effects of AJG049 and other Ca2+ channel antagonists (verapamil and diltiazem) on IBa at a holding membrane potential of −60 mV in guinea-pig mesenteric arterial myocytes. The concentration–response curve for AJG049 on IBa at −90 mV was also shown. The peak amplitude of IBa elicited by a step pulse to +10 mV from the holding potential (−60 or −90 mV) just before the application of drugs was normalized as one. The following values were used for curve fitting: AJG049, −60 mV, Ki=190.3 nM, nH=0.8; AJG049, −90 mV, Ki=1.9 μM, nH=0.8; verapamil, Ki=300.8 nM, nH=0.8; diltiazem, Ki=2.4 μM, nH=0.8. Each symbol indicates the mean of 4–8 observations with s.e.m. shown by vertical lines. Some of the s.e.m. bars are smaller than the size of the symbol. (d) Current–voltage relationships obtained in the absence (control) or presence of 100 nM AJG049. The current amplitude was measured as the peak amplitude of IBa in each condition. The lines were drawn by eye. (e) Relationship between the test potential and relative value of IBa inhibited by 100 nM AJG049, expressed as a fraction of the peak amplitude of IBa evoked by various amplitudes of depolarizing pulse in the absence of AJG049. Each symbol indicates the mean of five observations with ±s.e.m. shown by vertical lines. The line was drawn by eye.
Discussion
These experiments have demonstrated that AJG049 is a potent inhibitor of voltage-dependent L-type Ca2+ channels in guinea-pig ileal, colonic and vascular smooth muscle cells, and appears to inhibit the peak amplitude of voltage-dependent L-type Ca2+ currents in ileal myocytes with greater efficacy than those in vascular or colonic myocytes.
Inhibitory effects of AJG049 on voltage-dependent Ca2+ channels in smooth muscle
In ileal and mesenteric arterial myocytes, the inward ICa mainly flows through voltage-dependent L-type Ca2+ channels (Bean et al., 1986; Worley et al., 1986; Terada et al., 1987; Setoguchi et al., 1995). In the present study, nifedipine, a selective inhibitor of voltage-dependent L-type Ca2+ channels, suppressed Ca2+ (or Ba2+) currents in guinea-pig ileal and mesenteric arterial myocytes. We have shown that IC50 values for verapamil and diltiazem were very close to those of previous reports (Terada et al., 1987; Setoguchi et al., 1995).
In the present experiments, by use of radioligand receptor binding assays, AJG049 was shown to exhibit a high affinity for the diltiazem-binding site of voltage-dependent L-type Ca2+ channels. In conventional whole-cell recording, AJG049 suppressed both ICa in guinea-pig ileal myocytes and IBa in guinea-pig mesenteric arterial myocytes, showing concentration- and voltage-dependent characteristics. On removal of the drug, neither ICa in guinea-pig ileal myocytes nor IBa in guinea-pig mesenteric arterial myocytes recovered to control level. Furthermore, the rank order of the potency for inhibiting the peak amplitude of ICa (or IBa) coincides in guinea-pig ileal and mesenteric arterial myocytes (AJG049>verapamil>diltiazem) when conventional whole-cell recordings were made. These results strongly suggest that the target channel for AJG049 is likely to be the voltage-dependent L-type Ca2+ channel in guinea-pig ileal and mesenteric arterial myocytes, and that AJG049 exhibits a long-lasting inhibitory effect.
Kinetic studies concerning the actions of AJG049 on ICa
According to the receptor modulated hypothesis, each voltage-dependent Ca2+ channel is either in the resting, open or inactivated state (Hille, 1992). In the present study, the same amplitude of ICa was produced by the application of depolarizing pulses from a holding membrane potential of −90 mV or more negative, suggesting that all voltage-dependent Ca2+ channels at these potentials may be in the resting state (data not shown). The ability of AJG049 to suppress the peak amplitude of ICa evoked by a depolarizing pulse from two different holding potentials (−90 and −120 mV) was not significantly different, suggesting that at these negative holding potentials, AJG049 may inhibit ICa in a voltage-independent manner (AJG049: resting state block). When the holding potential was elevated to −60 mV from −90 mV, voltage-dependent inhibition by AJG049 was observed and the concentration–response curve was shifted to the left. The voltage-dependent inactivation curve was also shifted to the left after the application of AJG049. These results suggest that voltage-dependent inhibitory actions of AJG049 on voltage-dependent Ca2+ channels also occurred in the inactivated state of voltage-dependent Ca2+ channels (AJG049: voltage-dependent block). In the present experiments, the Krest value was estimated to be 739.1 nM from the concentration–response curve at a holding potential of −90 mV. When ΔVhalf value was obtained from the results using 10 s conditioning pulses, the estimated Kinact value was 120.4 nM (see Methods). Given this, we suggest that AJG049 may bind to the inactivated state with approximately six times higher affinity than to the resting state.
Tissue selectivity of AJG049 for voltage-dependent L-type Ca2+ channels in smooth muscle
The value of the selectivity ratio for AJG049 is the largest in the Ca2+ channel antagonists examined (AJG049 (2.9)>diltiazem (2.0) ≫verapamil (0.3)), although diltiazem, possessing profound cardiovascular effects in clinical field, also has a selectivity ratio of 2.0. Thus, it is somewhat difficult to categorize AJG049 as specific for intestinal Ca+ channels on this basis.
The observations also suggest that the binding affinity of AJG049 for inhibiting voltage-dependent Ca2+ channels may be different for vascular and intestinal smooth muscle.
Recently, molecular biological studies have revealed that the pore-forming structure of voltage-dependent Ca2+ channels is the α1 subunit and that the α1 subunit is a potential target for Ca2+ channel antagonists (reviewed by Slish et al., 1992). It is generally thought that the α1C subunit appears to be a pore-forming structure of L-type Ca2+ channels. Several isoforms of the α1C subunit of the L-type Ca2+ channel are expressed from different genes and produced by various splicing processes (Perez-Reyes and Schneider, 1995). Their relative expression is tissue-dependent and the relative expression of the major isoforms of α1C subunits, α1C-a (IVS3A, 46%) and α1C-b (IVS3B, 54%), in intestinal smooth muscles is different from that in vascular smooth muscles where the expression of the α1C-a (IVS3A) isoform is decreased to 14% of α1C subunits (Feron et al., 1994). Welling et al. (1997) reported that the tissue selectivity of nisoldipine was likely to be due to the difference in the affinities for the cardiac α1C-a subunit and the vascular α1C-b subunit. Similarly, it has been reported that nifedipine is a more potent inhibitor of the α1C-b subunit than the α1C-a subunit, although the potency of verapamil was identical with both isoforms (Morel et al., 1998). These results suggest that the diversity of α1C subunits might contribute to the diversity of responses of Ca2+ channels to Ca2+ channel blockers. Interestingly, different sensitivities of intestinal and vascular smooth muscles were commonly observed with several types of Ca2+ channel antagonists (Triggle et al., 1988; Godfraind et al., 1992; Zhang and Bolton, 1995). In the present experiments, AJG049 possessed an inhibitory action on L-type Ca2+ currents (diltiazem-binding site). Thus, we suggest that AJG049 readily binds with a high affinity to L-type Ca2+ channel isoforms.
Implications for gut-selective Ca2+ channel antagonists
Contractile activity of intestinal as well as vascular smooth muscle is related to the entry of Ca2+ from the extracellular fluid. As the bowel has a high rate of spontaneous activity under normal conditions, one of the main Ca2+ entry pathways is thought to be via voltage-dependent Ca2+ channels. In the clinical field, excessive postprandial non-propulsive contractions are commonly observed in IBS patients who have diarrhoea- and constipation-predominant disorders. Any blockade arising during spontaneous activity would be only slowly dissipated during a rest period; during hypermotility, its blocking action would be expected to develop strongly. Thus, as a wide variety of Ca2+ channel antagonists reduce abnormal motility of intestinal smooth muscle, Ca2+ channel antagonists are thought to be potentially useful drugs for the treatment of IBS. However, in patients with IBS, the activation of the autonomic nervous system induced by the hypotensive response associated with these drugs may adversely influence the symptoms (Aggarwal et al., 1994). In the present experiments, AJG049 showed some bowel selectivity, which may have been due to its use-dependent and persistent action on voltage-dependent L-type Ca2+ channels. We suggest that bowel-selective Ca2+ channel antagonists might be expected to be useful in conditions such as IBS, which is characterized by hypermotility of gut smooth muscle.
In conclusion, the present direct evidence indicates that AJG049 inhibits ICa (or IBa) in a long-lasting and voltage-dependent manner mainly through the diltiazem-binding site(s) of voltage-dependent L-type Ca2+ channels, and that any measurable gut selectivity exists only in certain regions of the digestive tract.
Acknowledgments
We thank Dr Frank R Edwards (Division of Neuroscience, John Curtin School of Medical Research, Australia National University, Canberra, Australia) for critically reading the manuscript. This work was supported by both a Grant-in-Aid for Scientific Research (B)-(2) from the Japanese Society for the Promotion of Science (Noriyoshi Teramoto, Grant Number 16390067) and a Grant-in-Aid for Exploratory Research (Noriyoshi Teramoto, Grant Number 17659075) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- AJG049
R-(+)-5,11-dihydro-5-[1-(4-methoxyphenethyl)-2-pyrrolidinylmethyl] dibenzo [b,e][1,4] oxazepine hydrochloride
- GF/C filters
glass microfibre filter type C
- IBa
voltage-dependent Ba2+ currents
- IBS
irritable bowel syndrome
- ICa
voltage-dependent Ca2+ currents
- PSS
physiological salt solution
- TEA
tetraethylammonium
Conflict of interest
The authors state no conflict of interest.
References
- Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, et al. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945–950. doi: 10.1016/0016-5085(94)90753-6. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, Sturek M, Puga A, Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986;59:229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- Byrne S. Verapamil in the treatment of irritable bowel syndrome. J Clin Psychiatry. 1987;48:388. [PubMed] [Google Scholar]
- Duridanova DB, Gagov HS, Boev KK. Two types of Ca2+-channels in cells from the circular layer of guinea-pig ileum. Gen Physiol Biophys. 1993;12:325–338. [PubMed] [Google Scholar]
- Feron O, Octave JN, Christen MO, Godfraind T. Quantification of two splicing events in the L-type calcium channel α1 subunit of intestinal smooth muscle and other tissues. Eur J Biochem. 1994;222:195–202. doi: 10.1111/j.1432-1033.1994.tb18857.x. [DOI] [PubMed] [Google Scholar]
- Godfraind T, Dessy C, Salomone S. A comparison of the potency of selective L-calcium channel blockers in human coronary and internal mammary arteries exposed to serotonin. J Pharmacol Exp Ther. 1992;263:112–122. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B.Calcium channels Ionic Channels of Excitable Membranes 1992Sinauer Associates Inc.: Massachusetts; 83–114.In: Hille B (ed)2nd edn [Google Scholar]
- Johnson D, Taylor Z, Gurney M, Volpe R, Cattau E., Jr Diltiazem versus placebo in treatment of patients with symptomatic irritable bowel syndrome. Am J Gastroenterol. 1988;83:1049. [Google Scholar]
- Lee HR, Roeske WR, Yamamura HI. High affinity specific [3H](+)PN 200-110 binding to dihydropyridine receptors associated with calcium channels in rat cerebral cortex and heart. Life Sci. 1983;33:1821–1829. doi: 10.1016/0024-3205(84)90340-0. [DOI] [PubMed] [Google Scholar]
- Morel N, Buryi V, Feron O, Gomez JP, Christen MO, Godfraind T. The action of calcium channel blockers on recombinant L-type calcium channel α1-subunits. Br J Pharmacol. 1998;125:1005–1012. doi: 10.1038/sj.bjp.0702162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y, Adachi N, Setoguchi M, Abe I, Fujishima M. Effects of CP-060S on membrane channels in vascular smooth muscle cells from guinea pig. Eur J Pharmacol. 1997;330:93–99. doi: 10.1016/s0014-2999(97)00173-8. [DOI] [PubMed] [Google Scholar]
- Perez-reyes E, Schneider T. Molecular biology of calcium channels. Kidney Int. 1995;48:1111–1124. doi: 10.1038/ki.1995.395. [DOI] [PubMed] [Google Scholar]
- Setoguchi M, Ohya Y, Abe I, Fujishima M. Inhibitory action of betaxolol, a β1-selective adrenoceptor antagonist, on voltage-dependent calcium channels in guinea-pig artery and vein. Br J Pharmacol. 1995;115:198–202. doi: 10.1111/j.1476-5381.1995.tb16339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slish D, Schultz D, Schwartz A. Molecular biology of the calcium antagonist receptor. Hypertension. 1992;19:19–24. doi: 10.1161/01.hyp.19.1.19. [DOI] [PubMed] [Google Scholar]
- Terada K, Kitamura K, Kuriyama H. Blocking actions of Ca2+ antagonists on the Ca2+ channels in the smooth muscle cell membrane of rabbit small intestine. Pflügers Arch. 1987;408:552–557. doi: 10.1007/BF00581155. [DOI] [PubMed] [Google Scholar]
- Teramoto N. Mechanisms of the inhibitory action of semotiadil fumarate, a novel Ca antagonist, on the voltage-dependent Ca current in smooth muscle cells of the rabbit portal vein. Jpn J Pharmacol. 1993;61:183–195. doi: 10.1254/jjp.61.183. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Yunoki T, Ikawa S, Takano N, Tanaka K, Seki N, et al. The involvement of L-type Ca2+ channels in the relaxant effects of the ATP-sensitive K+ channel opener ZD6169 on pig urethral smooth muscle. Br J Pharmacol. 2001;134:1505–1515. doi: 10.1038/sj.bjp.0704408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traube M, McCallum RM. Calcium-channel blockers and the gastrointestinal tract. Am J Gastroenterol. 1984;79:892–896. [PubMed] [Google Scholar]
- Triggle DJ, Hawthorn M, Zheng W. Potential-dependent interactions of nitrendipine and related 1,4-dihydropyridines in functional smooth muscle preparations. J Cardiovasc Pharmacol. 1988;12:S91–S93. doi: 10.1097/00005344-198806124-00018. [DOI] [PubMed] [Google Scholar]
- Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the α1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- Worley JFIII, Deitmer JW, Nelson MT. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci USA. 1986;83:5746–5750. doi: 10.1073/pnas.83.15.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bolton TB. Effects of UK-84149 on voltage-activated calcium currents of single smooth muscle cells from guinea-pig and rabbit jejunum and rabbit coronary artery. Br J Pharmacol. 1995;114:1657–1665. doi: 10.1111/j.1476-5381.1995.tb14954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]