Abstract
Background and purpose:
In this study we examined the effect of the natural product cardamonin, upon lipopolysaccharide (LPS)-induced inflammatory gene expression in order to attempt to pinpoint the mechanism of action.
Experimental approaches:
Cardamonin was isolated from the Greek plant A. absinthium L. Its effects were assessed on LPS-induced nitrite release and iNOS and COX-2 protein expression in two macrophage cell lines. Western blotting was used to investigate its effects on phosphorylation of the mitogen activated protein (MAP) kinases, ERK, JNK and p38 MAP kinase, and activation of the NFκB pathway, at the level of IκBα degradation and phosphorylation of NFκB. Also its effects on NFκB and GAS/GAF-DNA binding were assessed by EMSA.
Key results:
Cardamonin concentration-dependently inhibited both NO release and iNOS expression but had no effect on COX-2 expression. It did not affect phosphorylation of the MAP kinases, degradation of IκBα or phosphorylation of NFκB. However, it inhibited NFκB DNA-binding in both LPS-stimulated cells and nuclear extracts of the cells (in vitro). It also inhibited IFNγ-stimulated iNOS induction and GAS/GAF-DNA binding.
Conclusions and Implications:
These results show that the inhibitory effect of cardamonin on LPS-induced iNOS induction is not mediated via effects on the initial activation of the NFκB or MAP kinase pathways but is due to a direct effect on transcription factor binding to DNA. However, although some selectivity in cardamonin's action is implicated by its inability to affect COX-2 expression, its exact mechanism(s) of action has yet to be identified.
Keywords: cardamonin, TNFα, NO, Mitogen-activated protein kinase, NFκB
Introduction
Chalcones, a group of natural products widely distributed among plants, exhibit several therapeutic properties such as anti-cancer (Won et al., 2005), anti-inflammatory (Viana et al., 2003), antioxidant, antiviral (Uchiumi et al., 2003), antibiotic (Nielsen et al., 2004), antifungal (Jayasinghe et al., 2004) and antiallergic activities. The anti-inflammatory properties of some chalcones are attributed to their ability to inhibit the expression of the inducible enzymes NOS (iNOS) and cyclooxygenase-2 (COX-2), and thus the generation of nitric oxide (NO) and prostaglandin E2. In addition, they have been shown to suppress the production of proinflammatory cytokines such as tumour necrosis factor (TNFα) in activated macrophages (Herencia et al., 1999).
Cardamonin, a known 2′,4′-dihydroxy-6′-methoxychalcone, was isolated for the first time from Artemisia absinthium L. in this study. Cardamonin is known to have antiplatelet action in whole human blood (Dong et al., 1998) and an antimutagenic effect towards 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole in Salmonella typhimurium TA98 (Trakoontivakorn et al., 2001; Nakahara et al., 2002). Cardamonin also possesses appreciable in vitro anti-HIV activity (Tewtrakul et al., 2003). Furthermore, recent reports have demonstrated that cardamonin induces endothelium-dependent relaxation, primarily mediated through endothelial NO (Huang et al., 2000; Wang et al., 2001).
In order to extend the understanding of the anti-inflammatory actions of cardamonin, we investigated its effects in monocyte/macrophage cell lines stimulated with the Gram-negative bacterial cell wall component lipopolysaccharide (LPS). LPS acting via Toll-like receptor-4 is able to enhance the expression of a number of inflammatory genes such as iNOS and COX-2, principally through the activation of a number of transcription factors including activator protein-1 (AP-1) and cyclic AMP response element (CRE) and nuclear factor κB (NFκB). These factors are in turn regulated by members of the mitogen-activated protein (MAP) kinases, including extracellular signal-regulated kinase-1/-2 (ERK-1/ERK-2), p38 MAP kinase and c-Jun N-terminal kinase (JNK), and the inhibitory kappa B kinases (IKKs) which regulate NFκB translocation to the nucleus. Previously, we and others have shown the importance of these pathways in the expression of inflammatory proteins including iNOS and COX-2 (Paul et al., 1999; Bermejo-Gomez et al., 2005).
In this study, we show that cardamonin possesses potent anti-inflammatory properties by inhibiting LPS-induced iNOS expression and TNFα production in both RAW264.7 and a human monocytic cell line THP-1 cells, respectively. These effects were not owing to direct effects upon intermediates of either the MAP kinase or NFκB signalling cascades, but resulted from a nuclear effect involving inhibition of transcription factor binding to DNA.
Methods
Isolation of cardamonin
Cardamonin (Figure 1) was isolated for the first time from the flowers of A. absinthium L. obtained from Greece (voucher number: Hatziieremia 05/1, Royal Botanic Gardens of Edinburgh). Air-dried flowers (400 g) were ground to a powder and extracted in a Soxhlet apparatus using hexane, chloroform and methanol. The solvents were exchanged after 24 h of extraction, filtered and concentrated by rotary vacuum-evaporation at 40°C. The crude chloroform extract (29.3 g) was subjected to an initial fractionation by vacuum liquid chromatography (VLC) eluted with 100% petroleum ether and increasing the polarity by increments of 5% until 100% chloroform, then 10% methanol in chloroform until 100% methanol. Further fractionation of VLC fraction 20 obtained with 100% chloroform was performed using silica gel (Kieselgel 60 (0.063–0.020 mm)) open column chromatography (CC) and eluted isocratically with 9:1 ethyl acetate: petroleum ether. Preparative thin-layer chromatography (TLC) (Solvent system: 95:5 (v/v) CHCl3:CH3OH) of combined fractions 35–38 (72 mg) resulted in isolation of cardamonin. The compound was recrystallized by slow evaporation from methanol, and crystals were washed either with acetone or methanol. This process was repeated several times to yield 4.0 mg of the substance, which had a purity greater than 98%. The structural identity and purity of cardamonin was determined spectroscopically (13C and 1H NMR, MS) in comparison with previously published data (Itokawa et al., 1981) and redetermined by single-crystal X-ray diffraction (Synchrotron, Daresbury, UK).
Figure 1.
Chemical structure of cardamonin.
Cell culture conditions
THP-1 human monocytes and RAW264.7 murine macrophages were obtained from the European Cell Culture Collection and were maintained in Rosewell Park Memorial Institute medium 1640 and Dulbecco's modified Eagle's medium (DMEM), respectively, supplemented with 10% (v v−1) foetal calf serum (FCS), 2 mM glutamine and 250 IU ml−1 penicillin at 37°C in a humidified atmosphere of air/CO2 (19:1). Human skin epithelial cells NCTC2544 stably expressing PAR-2 were maintained in complete M199 medium with Earl's salt supplement (10% (v v−1) FCS, 100 U of penicillin ml−1, 100 μg of streptomycin ml−1) containing 400 μg ml−1 geneticin for selection and passaged using versene.
Enzyme-linked immunosorbent assay
THP-1 cells (1 ml) were seeded in 24-well plates at a concentration of 3 × 105 cells well−1 overnight before the addition of increasing concentrations of test agents or vehicle. LPS (1 μg ml−1) was added 30 min after the addition of compounds and incubated for a total of 4 h. Supernatants were removed and stored at −70°C until use. TNFα production was measured using a double-antibody enzyme-linked immunosorbent assay following manufacturer's protocol (R&D Systems, Oxon, UK). Briefly, a plate was coated with capture antibody 40 μg ml−1 (100 μl) overnight at 4°C. The plate was washed with 0.05% (v v−1) Tween-20 in phosphate-buffered saline (PBS). Blocking buffer consisting of 1% (w v−1) bovine serum albumin (BSA) and 5% (w v−1) sucrose in PBS was added for 2 h followed by repeated washing. A standard curve was set up consisting of rhTNFα at concentrations of 0–1000 pg ml−1. Supernatants were added in duplicate for 2 h at room temperature. Biotinylated detection antibody 200 ng ml−1 (100 μl), diluted in 0.05% (v v−1) Tween-20 and 0.1% (w v−1) BSA in Tris-buffered saline was incubated for 2 h, followed by washing and the addition of streptavidin–horseradish peroxidase (HRP) (1:4000 dilution) for 20 min. The plate was washed and substrate solution added for 30 min. The reaction was stopped with 10% (v v−1) H2SO4 and the plate was read on a microplate reader (UVSpectramax, Molecular Devices) at 450 nm in comparison to a standard curve constructed with TNFα (1–1000 nM).
Measurement of NO production
NO production was measured in RAW264.7 macrophages as nitrite production (NO2−). Cells were grown until near confluent in a 12-well plate. Cells were pretreated with cardamonin alone or cardamonin for 30 min followed by LPS for 12 h or interferon gamma (IFNγ) (100 International unit (IU) ml−1). Supernatants (50 μl) were removed and transferred onto 96-well plates mixed with equal amounts of Griess reagent (1:1 mixture (v v−1) of 2% (w v−1) sulphanilamide and 0.2% (w v−1) naphthylenediamine dihydrochloride in 5% (v v−1) H3PO4) and left for 10 min at room temperature. The optical density was measured on a microplate reader (SpectraMax 190, Molecular Devices) at 540 nm.
Western blotting
THP-1 (1 ml) (4 × 105 ml−1) or RAW264.7 cells were treated with cardamonin or vehicle for 30 min before treatment with LPS (1 μg ml−1) or IFNγ (100 IU ml−1). Cells were washed twice in ice-cold PBS and lysed by adding 0.5 ml of preheated (70°C) Laemmli sample buffer (63 mM Tris-HCl (pH 6.8), 2 mM Na4P2O7, 5 mM ethylenedinitrilo-N, N, N′, N′-tetraacetate (EDTA), 10% (v v−1) glycerol, 2% (w v−1) sodium dodecyl sulphate and 0.0007% (w v−1) bromophenol blue and 50 mM dithiothreitol (DTT)). The whole-cell lysates were boiled for 3 min and stored until use at −20°C. For iNOS and COX-2, 10 μg of total cellular protein was determined by Bradford reaction, and subjected to 7.5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted onto nitrocellulose. For phosphorylated MAPKs, p65 and IκBα cell lysates were subjected to 10% SDS–PAGE. The blots were blocked for nonspecific binding for 2 h in 50 mM Tris-HCl buffer (pH 7.4), 150 mM NaCl, 0.2% (v v−1) Tween-20, (NaTT), containing 2% (w v−1) BSA. Blots were then incubated overnight in 0.2% (w v−1) BSA/NaTT with either 1 μg ml1 MAP kinase, IκBα, phosphorylated-p65, iNOS or COX-2 antibodies. Blots were washed in NaTT for 90 min before being incubated for 2 h in 0.2% (w v−1) BSA/NaTT with either HRP-conjugated anti-rabbit or anti-mouse IgG antibody. Following further washing (90 min), blots were developed using enhanced chemiluminescence reagents.
Electrophoretic mobility shift assay preparation of nuclear extracts and assay of NFκB-DNA-binding activity
RAW264.7 cells were grown on six-well plates and exposed to cardamonin and vehicle (0.3% (v v−1) dimethyl sulphoxide) for 30 min before stimulation with LPS (2 h) or IFNγ (2 h). All procedures for nuclear protein extraction were conducted on ice. Cells were washed twice and scrapped into 1 ml of PBS and pelleted at 13 000 r.p.m. for 1 min. The pellet was resuspended in 400 μl of Buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM phenylmethylsulphonylfluoride (PMSF) and 10 μg ml−1 of aprotinin, leupeptin and pepstatin) and incubated on ice for 15 min. NP-40 (25 μl) (10% w v−1) was added to each sample and vortexed for 10 s before centrifugation for 1 min at 13 000 r.p.m. The pellet was resuspended in 20 μl Buffer B (20 mM HEPES, 25% (v v−1) glycerol, 0.4 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF and 10 μg ml−1 of aprotinin, leupeptin and pepstatin), vortexed and shaken at 4°C for 30 min. The samples were then sonicated (2 × 30 s). Supernatants were collected following centrifugation at 13 000 r.p.m. for 30 min and protein contents were determined by Bradford assay. The samples were stored at −80°C until used for electrophoretic mobility shift assay (EMSA).
The oligonucleotide probes used for EMSA contained the NFκB consensus sequence: 5′-AGTTGAGGGGACTTTCC CAGGC-3′, or the γ-activated site (GAS)/γ-activating factor (GAF) consensus sequence: 5′-AGCCTGATTTCCCCGAAAT GACGGC-3′. 32P-γ-ATP was used to label the oligonucleotide at its 5′-end by incubation with T4 polynucleotide kinase at 37°C for 30 min. The reaction was terminated by the addition of 0.5 M EDTA and the labelled oligonucleotide diluted in TE buffer (10 mM Tris base (pH 8.0), 1 mM EDTA). The efficiency of 32P-phosphate incorporation into the oligonucleotide was determined by scillintation counting.
Nuclear protein (5 μg), a total volume of 9 μl binding buffer containing 10 mM Tris-HCl (pH 7.5), 4% (v v−1) glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 50 μg ml−1 poly-[dI–dC.dI–dC] was incubated for 30 min before the addition of 1 μl (50 000 c.p.m.) of 32P-labelled oligonucleotide probe for 30 min. Following incubation, 1 μl of 10 × loading buffer (250 mM Tris-HCl (pH 7.5), 0.2% (w v−1) bromophenol blue, 40% (v v−1) glycerol) was added to the samples and DNA–protein complexes were separated from the unbound probe by non-denaturing electrophoresis on 5% (w v−1) polyacrylamide gels in 0.5 × TBE running buffer at 100 V. After electrophoresis, the gels were dried and visualized by autoradiography. The specificity of the binding reaction was examined by competitive analysis with a 25-fold molar excess of unlabelled oligonucleotide probe in the binding reaction.
Luciferase gene reporter assay
NCTC2544 cells stably expressing PAR-2 (Clone G) (Kanke et al., 2001) were stably co-transfected with either NFκB, AP-1 or CRE luciferase constructs and cells grown in 96-well plates until near confluent and rendered quiescent for 18 h in serum-free medium. Cells were treated with increasing concentrations of cardamonin for 30 min before the addition of appropriate concentrations of trypsin and phorbol myristate (PMA) for 6 h. Stimulation was terminated by aspiration of the medium, washing with ice-cold PBS and addition of luciferase solution (50 μl of 1 mg luciferase powder in 5 ml of lysis buffer (25 mM Tris phosphate (pH 7.90), 8 mM MgCl2, 1 mM DTT, 1% (v v−1) Triton X-100, 15% (v v−1) glycerol) in each well and the plate was read on a Wallac Trilux 1450 microbeta counter.
Cell viability assay
Toxicity of cardamonin in RAW264.7 and THP-1 cells was assessed using an Alamar Blue™ Reduction assay. Cells (100 μl) (2 × 105cells well−1) were seeded in 96-well plates in appropriate medium containing 10% (v v−1) FCS. Next day, medium was aspirated and replaced with medium containing serial dilutions of cardamonin and 10% (v v−1) Alamar Blue™. Cells in medium alone, medium plus vehicle and water were used as negative controls. After incubation with the test agents for 24 h, the medium was replaced in all wells with fresh medium containing 10% (v v−1) Alamar Blue™. Half of the negative control wells were replaced with water and Alamar Blue™. Measurements of reduction of Alamar Blue™ were taken as absorbances at 570 and 595 nm, both at 24 and 48 h after initial addition of cardamonin, using a microplate reader (UVSpectramax, Molecular Devices). Results were verified using a Trypan Blue dye exclusion assay.
Statistical analysis
Data are presented as means±s.e.mean of the indicated number of experiments. Statistical comparisons between groups were performed using one-way ANOVA, followed by the Dunnett's post hoc test or the Student's t-test as appropriate. Differences between means were considered significant when P<0.05.
Materials
All materials used were of the highest grade available and were purchased from Sigma-Aldrich Co. Ltd (Poole, Dorset, UK) or VWR International Ltd (Poole, UK), unless otherwise stated. DMEM was purchased from Gibco (Paisley, UK). Monoclonal anti-human TNFα antibody and streptavidin HRP were purchased from R&D Systems (Oxon, UK). HRP-conjugated sheep anti-mouse IgG and HRP-conjugated donkey anti-rabbit IgG were purchased from Amersham Pharmacia Biotech Inc. (Piscataway, NJ, USA). Antibodies against p38, IκBα, iNOS, COX-2 and phosphospecific antibodies p38, ERK1–ERK2, JNK and p65 were obtained from Insight Biotechnology (London, UK). Oligonucleotides were purchased from Promega Co. (Southampton, UK) and Sigma-Genosys Ltd (Cambridge, UK).
Results
Cardamonin inhibits LPS-induced TNFα production in THP-1 human monocytes
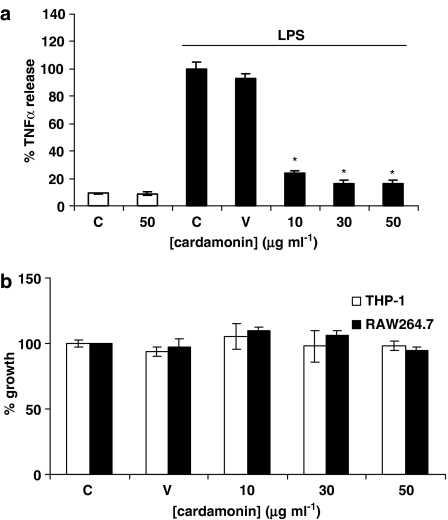
To investigate the potential anti-inflammatory effects of cardamonin, we examined first its effects on LPS-induced TNFα production in a human monocytic cell line THP-1. Exposure of THP-1 cells to LPS (1 μg ml−1) for 4 h produced a 12- to 18-fold increase in TNFα production when compared to basal levels in the absence of LPS. The addition of cardamonin 30 min before LPS stimulation markedly inhibited TNFα production in a concentration-dependent manner (IC50=9.12±1.12 μg ml−1, n=4) (Figure 2a), Cardamonin pretreatment alone did not have any effect on basal TNFα production from THP-1 cells, although over a similar concentration range cardamonin was found to have little effect on THP-1 cellular viability and metabolism (Figure 2b).
Figure 2.
Effect of cardamonin on LPS-stimulated TNFα production in THP-1 monocytes and on cell viability of the human monocytes THP-1 and murine macrophages RAW264.7. In (a), THP-1 monocytes were pretreated with vehicle (V) or increasing concentrations (10–50 μg ml−1) of cardamonin for 30 min before stimulation with LPS (1 μg ml−1) for 4 h. TNFα production was then measured as described in Materials and methods. Each value is the % mean±% s.e.mean of three experiments, *P<0.05 from LPS. (control+cardamonin alone=8.18±1.8 pg ml−1). In (b), cells were treated with 50, 30 and 10 μg ml−1 of cardamonin for 24 h. Cell viability was measured as outlined in Materials and methods. Results are expressed as the mean±s.e.mean for three independent experiments; *P<0.05, significantly different from control (C).
Cardamonin inhibits LPS-induced iNOS protein expression and NO production with no effect on COX-2 protein in RAW264.7 murine macrophages
To examine whether cardamonin affected the release of other inflammatory chemical mediators, the effects of cardamonin on NO production, iNOS and COX-2 protein expression were investigated (Figure 3). The time-dependent effect of LPS (1 μg ml−1) on NO production, measured as nitrite production, was investigated over the period of 36 h (data not shown). Significant NO production was observed at 12 h (5.6±2.2 fold increase) after stimulation, with maximal production at 24 h (16.8±1.0 fold increase) and maintained at 36 h (data not shown). NO production from LPS-stimulated RAW264.7 cells (12 h) was inhibited in a concentration-dependent manner by cardamonin (IC50=9.39±0.56 μg ml−1, n=3), with the highest concentrations of 10–50 μg ml−1 abolishing the effect of LPS (Figure 3a).
Figure 3.
Effect of cardamonin on LPS-induced COX-2 and iNOS protein levels and NO production in RAW264.7 macrophages. RAW264.7 macrophages were pretreated with vehicle (V) or increasing concentrations (1–50 μg ml−1) of cardamonin for 30 min before stimulation with LPS (1 μg ml−1) for 12 h. (a) Nitrite production was analysed in the cell supernatants using a modified Griess reaction. (b and c) Whole-cell extracts were assayed for iNOS and COX-2 expression as described in Materials and methods. Quantification of Western blot was performed by scanning densitometry. Each blot is representative of three others. Each value is the mean±s.e.mean of three experiments, *P<0.05 from LPS control (C).
The inhibitory effects of cardamonin on NO release correlated with effects on iNOS expression, assessed by Western blotting (Figure 3b). Pretreatment with increasing concentrations of cardamonin before LPS stimulation inhibited LPS-induced iNOS induction (IC50=13.29±1.88 μM, n=3) over a similar concentration range to nitrite production (Figure 3b). These data demonstrate that the inhibitory capacity of cardamonin against NO production is probably the result of inhibition of iNOS protein expression in LPS-treated RAW264.7 macrophages.
In contrast, cardamonin had no effect on LPS-induced COX-2 protein expression measured after 12 h of exposure to LPS (Figure 3c). Over the concentration range of 10–50 μg ml−1 cardamonin, no significant changes were observed in the level of protein expression. In addition, in non-stimulated cells, the presence of cardamonin alone increased COX-2 protein levels by approximately two-fold.
Cardamonin does not affect LPS-induced MAPK phosphorylation in THP-1 monocytes and RAW264.7 macrophages
The effect of increasing concentrations of cardamonin on maximal LPS stimulation of the MAP kinases, ERK, p38 MAP kinase and JNK were examined in both THP-1 cells and RAW264.7 macrophages. Pretreatment with cardamonin had no effect on the phosphorylation of either p38, ERK1/2 or JNK (Figure 4I–II). However, in unstimulated cells, treatment with cardamonin resulted in an increase in two-fold of the phosphorylation of p38 in both THP-1 monocytes and RAW264.7 macrophages (Figure 4Ia and IIe).
Figure 4.
Effect of cardamonin on LPS-induced MAP kinases in (I) THP-1 monocytes and (II) RAW264.7 macrophages. Cells were pretreated with either vehicle (V) or increasing concentrations of cardamonin (10–50 μg ml−1) alone or before stimulation with LPS (1 μg ml−1) for 30 min. Cell lysates were prepared and assayed for phospho-p38 (a and e) and total p-38 (d and h), phospho-ERK1/2 (c and g) and phospho-JNK (b and f). These blots are representative of three others.
Cardamonin does not affect LPS-induced IκBα degradation and phosphorylation of p65 in THP-1 monocytes and RAW264.7 macrophages
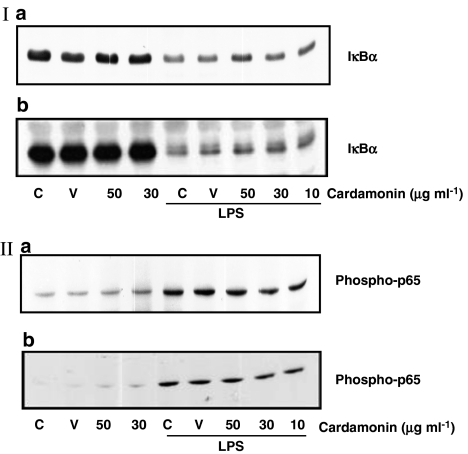
Exposure of both RAW264.7 macrophages and THP-1 monocytes to 1 μg ml−1 LPS led to substantial loss of IκBα and increase in phosphorylated levels of NFκB subunit, p65 in a time-dependent manner (data not shown). In both cells, LPS-stimulated IκBα degradation was maximal after 30 min of exposure to LPS and returned to basal levels after 90 min, whereas an increase in phosphorylated levels of p65 was observed 10 min after exposure and sustained up to 90 min (results not shown). However, again pretreatment of cells with cardamonin in the presence of LPS was without effect on either IκBα (Figure 5Ia and b) or phosphorylated p65 levels (Figure 5a and b) in RAW 264.7 macrophages and THP-1 monocytes. In contrast to results observed for p38 MAP kinase, cardamonin had no effect on either parameter alone.
Figure 5.
Effect of cardamonin on LPS-induced loss of IκBα and phosphorylation of p65 in THP-1 monocytes and RAW264.7 macrophages. THP-1 monocytes (I and II, a) and RAW264.7 macrophages (I and II, b) were pretreated with either vehicle (V) or increasing concentrations of cardamonin (10–50 μg ml−1) alone before stimulation with LPS (1 μg ml−1) for 30 min. Cell lysates were prepared and assayed for IκBα (I, a and b) and phosphorylated-p65 (II, a and b) using the specific antibodies as described in Materials and methods. These blots are representative of three others.
Cardamonin interferes with LPS-induced binding of NFκB to DNA without inhibiting its nuclear translocation in RAW264.7 cells
Cardamonin was then examined to see if it would inhibit the binding of NFκB to DNA by EMSA. As shown in Figure 6a, LPS (1 μg ml−1) treatment for 2 h significantly increased NFκB-DNA-binding activity. This is consistent with data from our previous studies (Paul et al., 1999). Treatment with cardamonin for 30 min before the addition of LPS markedly inhibited the DNA-binding of p65 NFκB. As we have already shown that the inhibitory effect of cardamonin on DNA-binding activity of NFκB is not owing to NFκB inactivation, we hypothesized that cardamonin might inhibit NFκB-DNA binding by inhibiting nuclear translocation of NFκB. Western blot analysis of the nuclear extracts (Figure 6b) showed that cardamonin had no effect upon the LPS-induced increased levels of p65 into the nucleus, thus revealing that cardamonin is acting at the level of DNA binding rather than preventing p65 nuclear translocation. Further work confirmed that adding cardamonin for 30 min to the EMSA mixture before the addition of oligonucleotide was capable of attenuating the NFκB-DNA binding over a similar range to that observed in whole-cell studies (Figure 6c). Hence, it was confirmed that cardamonin disrupts the interaction of this transcription factor with its consensus binding sites.
Figure 6.
Effect of cardamonin on the nuclear translocation of NFκB and DNA-NFκB binding activity in RAW264.7 macrophages following exposure to LPS. Cells were stimulated with 1 μg ml−1 of LPS for 1 h after pretreatment with either vehicle (V) or increasing concentrations of cardamonin. Nuclear extracts were prepared as described in Materials and methods. (a) Effect of cardamonin on DNA-NFκB binding activity as measured by EMSA and presented the relative amounts of binding as fold induction of the density of the bands quantified by scanning densitometry. (b) Effect of cardamonin on LPS-induced levels of p65/NFκB in the nucleus as measured by Western blotting. (c) Effect of addition of cardamonin to previously prepared nuclear extracts on NFκB-DNA-binding activity. Results are expressed as the mean±s.e.mean for three independent experiments; *P<0.05 vs control (C).
Cardamonin inhibits trypsin- and PMA-induced NFκB, AP-1 and CRE transcriptional activities in Clone G cells
To investigate whether the inhibitory effect of cardamonin upon NFκB-DNA binding was also observed at the level of transcriptional activity and to test whether its effects were cell type and/or gene specific, we employed a luciferase reporter assay using human skin epithelial-derived Clone G cells stably expressing both the proteinase-activated receptor, PAR-2, and either NFκB, AP-1 or CRE (MacFarlane et al., 2005). Treatment with PMA (10 nM) and trypsin (30 nM) for 6 h stimulated NFκB transcription by 26.8±0.7- and 6.77±0.5-fold over the baseline levels (Figure 7a). Pretreatment of cells with cardamonin inhibited both PMA and trypsin-induced NFκB transcription with IC50 values of 5.6±0.2 μM and 12.17±1.2 μg ml−1. Treatment with PMA (30 nM) and trypsin (50 nM) for 6 h stimulated AP-1 transcription by 4.2±0.6- and 6.8±0.7-fold over baseline, respectively. Furthermore, pretreatment of cells with cardamonin inhibited both the PMA and trypsin-induced AP-1 transcription with IC50 values of 1.6±0.7 and 13.4±3.6 μg ml−1, respectively (Figure 7b). Treatment with PMA (100 nM) and trypsin (100 nM) for 6 h stimulated CRE transcription by 19.6±2.8- and 6.8±0.4-fold over baseline. The PMA and trypsin-induced CRE transcriptional activity was also inhibited by pretreatment of cells with cardamonin with IC50 values of 0.7±2.4 and 8.3±0.8 μg ml−1, respectively (Figure 7c).
Figure 7.
Concentration-dependent inhibition by cardamonin on PMA and trypsin-induced stimulation of NFκB (a), AP-1 (b) and CRE (c) transcriptional activity in Clone G cells. Cells were grown to near confluency and rendered quiescent for 18 h. Cells were treated with appropriate concentrations of agonists in the presence of increasing concentrations of cardamonin. Cells were assayed as described in Methods and materials. Each value represents the mean±s.e.mean of three experiments, *P<0.05 from PMA- or trypsin-control.
Cardamonin inhibits IFNγ-induced iNOS protein expression and NO production by inhibiting the GAS/GAF-DNA-binding activity in RAW264.7 murine macrophages
In addition, we measured the effect of cardamonin on iNOS expression and NO production induced by IFNγ, a cytokine that induces inflammatory gene expression but via a different pathway than that for LPS. RAW264.7 cells were treated over the same time period with IFNγ (100 IU ml−1) as with LPS. Pretreatment of cells with cardamonin for 30 min before the addition of inhibited the iNOS expression (IC50=11.3±1.1 μg ml−1, n=3) (Figure 8a) and NO production (IC50=15.6±1.8 μg ml−1, n=3) (Figure 8b) with similar potencies than that observed with LPS stimulation.
Figure 8.
Effect of cardamonin on IFNγ-induced iNOS protein levels and GAS/GAF-DNA-binding activity in RAW264.7 macrophages. (a and b) RAW264.7 macrophages were pretreated with vehicle (V) or increasing concentrations (1–50 μg ml−1) of cardamonin for 30 min before stimulation with IFNγ (100 IU ml−1) for 12 h. Whole-cell extracts were assayed for iNOS expression (a) and nitrite production (b) as described in Materials and methods. (c) RAW264.7 macrophages were pretreated with vehicle or increasing concentrations (1–50 μg ml−1) of cardamonin for 30 min before stimulation with IFNγ (100 IU ml−1) for 2 h. Nuclear cell extracts were assayed for GAS/GAF-DNA binding as described in Materials and methods. Quantification of Western blot and EMSA was performed by scanning densitometry. Each blot is representative of three others. Each value is the mean±s.e.mean of three experiments, *P<0.05 from IFNγ control (C).
An important transcription factor mediating the rapid response to IFNγ is known to be the GAF, and its association with the γ-activated site (GAS) leads to transcriptional activation of the interferon regulatory factor-1 (IRF-1) (Liu et al., 2001). The role of IRF-1 in the induction of iNOS gene is well established (Upreti et al., 2004). As cardamonin was shown to affect the transcriptional activation of iNOS gene induced by LPS, we hypothesized that its action might be similar in the IFNγ pathway. Therefore, we tested the effect of cardamonin on IFNγ-induced GAS/GAF-DNA-binding activity. As shown in Figure 8c, IFNγ (100 IU ml−1) treatment for 2 h significantly increased GAS/GAF-DNA-binding activity. Treatment with cardamonin for 30 min before the addition of IFNγ inhibited markedly the DNA binding of GAS/GAF over the same concentration range observed for effects upon NFκB-DNA binding.
Discussion
The present study provides evidence that cardamonin, a known chalcone isolated for the first time from the dried flowers of A. absinthium L., possesses anti-inflammatory properties by inhibiting TNFα production in THP-1 monocytes and synthesis of NO in RAW264.7 macrophages, both factors that contribute to the onset of inflammatory pathophysiological diseases such as septic shock, infection and cancer. Furthermore, the inhibitory effect of cardamonin on NO synthesis is correlated to its inhibitory effect on iNOS, the enzyme responsible for the conversion of L-arginine to NO in macrophages. Several studies have shown that synthesized hydroxylated chalcones are capable of inhibiting LPS/IFNγ-induced NO production in a concentration-dependent manner in murine RAW264.7 macrophages (Ko et al., 2003; Won et al., 2005). Recent studies though have revealed that hydroxychalcones such as the naturally occurring broussochalcone A and butein as well as the synthetic derivative 3′,4′,5′,3,4,5-hexamethoxy-chalcone abrogate LPS-induced iNOS and COX-2 expression as well as TNF-α release in RAW264.7 macrophages by inhibiting the LPS-induced degradation of the inhibitory protein IκBα and thus the translocation of the transcription factor NF-κB to the nucleus (Cheng et al., 2001; Lee et al., 2004; Alcaraz et al., 2004). These data suggest that chalcones may have a common site and mechanism of action.
LPS induced iNOS induction has previously been shown to be dependent on the activation of NFκB (Lowenstein et al., 1993; Peng et al., 1995) and more recently activation of the IKKs (Ghosh and Karin, 2002; Bermejo-Gomez et al., 2005). Nevertheless, our studies showed that cardamonin has a different site and mechanism of action than the reported anti-inflammatory hydrochalcones. We found that cardamonin has little effect on IκBα degradation, suggesting that the effect of this chalcone is unlikely to be at the level of IKKβ activation and subsequent phosphorylation, ubiquination and degradation of IκBα (Ghosh and Karin, 2002). We also measured the effect of cardamonin on another parameter, the phosphorylation of p65 NFκB, an event that is also known to be regulated, in part, by IKK (Mattioli et al., 2004; Wang and Baldwin, 1998). Our results therefore contrast with the actions of a number of chalcones and other natural products that have, as a site of action, the IKKs. This includes the widely distributed flavonoids quercetin and luteolin (Kim and Jobin, 2005; Chen et al., 2005), which represent novel anti-inflammatory compounds and may have increased potency relative to the current commercial inhibitors such as SC514 (Bermejo-Gomez et al., 2005). However, the results in this study also contradict the recently published findings of Lee et al. (2006), which show that cardamonin, at a concentration as low as 30 μM (∼8 μg ml−1), completely inhibits IκBα degradation and phosphorylation of p65/NFκB in RAW264.7 macrophages, an effect mediated via inhibition of IKK. We observed no such effect on either IκBα degradation or phosphorylation of p65 NFκB at these concentrations. A much higher concentration of 50 μg ml−1 (∼185 μM) was required for the nuclear-derived effects. Purity is essential in testing natural products and minor impurities can either potentiate the pharmacological behaviour of a compound in a synergistic manner or alter its profile. In the work of Lee et al. (2006), no information on the purification of the chalcone was presented.
Several reports have shown that the LPS signalling cascade leading to TNFα production in macrophages and monocytes is dependent on the activation of the members of the MAP kinase family: p38, ERK1/2 and JNK (Bruggen et al., 1999; Swantek et al., 1997; Anderson and Sundler, 2000). For example, ERK1/2 and p38 activation upregulates LPS-induced COX-2 expression, but not iNOS in murine RAW264.7 cells (Paul et al., 1999), whereas in J744 murine macrophages, it has been shown that these MAP kinases are also involved in both LPS- and LPS/IFNγ-induced iNOS expression (Chen et al., 1999; Lahti et al., 2000; Chan and Riches, 2001). Again, cardamonin pretreatment of both THP-1 and RAW264.7 cells had no effect on the LPS activation of the members of MAPK family. In fact, there was a small but consistent increase in the activation of p38 MAP kinase that was reflected in the increase in COX-2 induction. This finding is in accordance with previous reports demonstrating the link between p38 activation and COX-2 induction as a result of the partial blockade of COX-2 expression by the p38 pharmacological inhibitor SB203580 in murine RAW264.7 macrophages (Hwang et al., 1997; Paul et al., 1999). Several natural products have been shown to inhibit the expression of these genes by modulating the phosphorylation of MAPK pathways. For example, luteolin inhibits LPS-induced TNFα production in RAW264.7 cells by simultaneous inhibition of the ERK1/2 and p38 pathways (Xaragori et al., 2002). Similarly, the LPS-increased iNOS expression was significantly diminished by pretreatment with the chalcone butein in RAW264.7 cells and this was partly explained by the reduction in phosphorylation of ERK1/2 (Lee et al., 2004). However, a recent study of the inhibitory actions of 2′-hydroxychalcones on agonist-stimulated iNOS and TNFα expression in RAW264.7 macrophages demonstrated a role for JNK (Ban et al., 2004).
Transcriptional control of the murine iNOS gene has been well characterized and contains several binding sites for transcriptional factors such as NFκB, AP-1 and various members of the CCAAT/enhancer-binding protein family (Lowenstein et al., 1993; Xie et al., 1994). Several previous studies have implicated NFκB as a critical factor for the transcriptional response of iNOS gene to LPS in macrophages (Xie et al., 1994). The transcriptional activation of the human TNFα gene seems rather more complex, containing binding sites for NF-κB, AP-1, CREB, Ets, Sp1 and Elk-1 (Tsai et al., 2000; Vallejo et al., 2000). As none of the common pathways involved in the regulation of TNFα and iNOS was found to be affected by pretreatment with cardamonin, a number of other parameters were assessed. Cardamonin was found to inhibit NFκB-DNA binding in LPS challenged macrophages. This effect was found to be within the range observed for the inhibitory effect upon iNOS induction by LPS. From these results in combination with those obtained by Western blotting of the nuclear extracts that showed that NFκB translocation to the nucleus was not affected, it was assumed that the site was within the nucleus itself. This assumption was verified by immunofluorescent studies, which demonstrated no change in the levels of p65 NFκB for cardamonin-treated RAW264.7 macrophages compared to those only challenged with LPS (data not shown). Furthermore, preincubation of nuclear extracts with cardamonin also showed that the effect upon NFκB-DNA-binding and on other transcription factors is direct. Previous unpublished work has shown that in RAW264.7 macrophages, the NFκB complex consists of p65 and Rel-C (results not shown). As cardamonin pretreatment abolished NFκB-DNA binding, this suggests that there is no selectivity in the effect of cardamonin on these isoforms.
Furthermore, these findings were consistent with the observed inhibitory effects of cardamonin on the reporter activity for NFκB, AP-1 and CRE DNA-binding and transcriptional activation, suggesting that the effect of cardamonin inhibits transcription factor binding to consensus binding sites within numerous genes. Although this is the first report that cardamonin might disrupt the binding of transcription factors to DNA, several studies have depicted a similar behaviour from molecules characterized as binders to the major and minor grooves of DNA. For example, the antibiotic distamycin A, a minor groove binder that can inhibit transcription factor binding to AT-rich regions of DNA, was recently reported to selectively inhibit only the interaction of IRF-1 with the interferon-stimulated response element binding site with the murine NOS2 promoter (Baron et al., 2004).
We further extended our studies on the effect of cardamonin on the IFNγ-induced signalling pathway. IFNγ challenge in macrophages leading to iNOS expression has been demonstrated to lead to the activation of the Janus kinase (JAK1/2), phosphorylation of the cytoplasmic transcription factor STAT1α and formation of homodimers that eventually translocate into the nucleus to bind to the distinct IFNγ-responsive promoter region known as γ-activating sequences (GAS) (Stark et al., 1998). Cardamonin also inhibited the IFNγ-induced iNOS expression and NO production in RAW264.7 macrophages; this was reflected in an inhibition of GAS/GAF–DNA complex formation. Again these results suggest that the selectivity of the effects of cardamonin is not at the level of transcription factors but is within the DNA.
Currently, we have not identified the exact mechanism of action of cardamonin. The compound is clearly not a general inhibitor of gene induction as it failed to inhibit the expression of COX-2 protein. The inhibitory property on multiple transcription factor binding to DNA might arise from the fact that cardamonin might act as a minor groove binder with selective behaviour against certain genes or might inhibit the attachment of certain transcription factors to the double helix by selective direct binding onto amino-acid residues of the transcription factors. Sesquiterpene lactones (SLs) are a group of natural products that exhibit the latter behaviour; their anti-inflammatory activity is associated with the modulation of the p65/NFκB DNA complex (Lyss et al., 1997, 1998; Rungeler et al., 1998; Kwok et al., 2001). Moreover, the SLs helenalin and parthenolide were shown to alkylate p65/NFκB at Cys38 when the Cys38 → Ser mutant was unaffected compared to the wild-type p65/p65 DNA complex (Garcia-Pineres et al., 2001). Further studies using labelled cardamonin or capillary gel electrophoresis may help to elucidate the mechanism of action of this compound at the level of DNA binding and transactivation.
Conflicts of interest
The authors state no conflict of interest.
Acknowledgments
This work was sponsored by a Greek Ministry of Health and Social Solidarity studentship to SH.
Abbreviations
- AP-1
activator protein-1
- BSA
bovine serum albumin
- COX-2
cyclooxygenase-2
- CRE
cyclic AMP response element
- DMEM
Dulbecco's modified Eagle's medium
- DTT
dithiothreitol
- EDTA
ethylenedinitrilo-N, N, N′, N′-tetraacetate
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun kinase
- FCS
foetal calf serum
- GAF
γ-activated factor
- GAS
γ-activated site
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- HRP
horseradish peroxidase
- IFNγ
interferon gamma
- IκBα
inhibitory kappa B alpha
- IKK
IκB kinase
- iNOS
inducible NOS
- IRF-1
IFN regulatory factor
- LPS
lipopolysaccharide
- MAP kinase
mitogen-activated protein kinase
- NFκB
nuclear factor kappa B
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PMA
phorbol myristate
- PMSF
phenylmethylsulphonylfluoride
- STAT
signal transducer and activator of transcription
- TNFα
tumour necrosis factor alpha
References
- Alcaraz MJ, Vicente AM, Araico A, Dominguez JN, Terencio MC, Ferrandiz ML. Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J Pharmacol. 2004;142:1191–1199. doi: 10.1038/sj.bjp.0705821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, Sundler R. Signaling to translational activation of tumour necrosis factor-α expression in human THP-1 cells. Cytokine. 2000;12:1784–1787. doi: 10.1006/cyto.2000.0784. [DOI] [PubMed] [Google Scholar]
- Ban HS, Suzuki K, Lim SS, Jung SH, Lee HS, Lee YS, et al. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2′-hydroxychalcone derivatives in RAW 264.7 cells. Biochem Pharmacol. 2004;67:1549–1557. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Baron RM, Carvajal IM, Liu X, Okabe RO, Fredenburgh LE, Macias AA, et al. Reduction of nitric oxide synthase 2. Expression by distamycin A improves survival from endotoxemia. J Immun. 2004;173:4147–4153. doi: 10.4049/jimmunol.173.6.4147. [DOI] [PubMed] [Google Scholar]
- Bermejo-Gomez A, MacKenzie C, Paul A, Plevin R. Selective inhibition of inhibitory kappa B kinase-beta abrogates induction of nitric oxide synthase in lipopolysaccharide-stimulated rat aortic smooth muscle cells. Br J Pharmacol. 2005;146:217–225. doi: 10.1038/sj.bjp.0706308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggen VDT, Ninjenhius S, Raaij VE, Verhoef J, Asbek BSV. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the Raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 1999;67:3824–3829. doi: 10.1128/iai.67.8.3824-3829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Riches DW. IFN-gamma+LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(MAPK) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280:C441–C450. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–129. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Ho FM, Pei-Dawn LC, Chen CP, Jeng KC, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Lin C, Hwang T, Teng C. Broussochalcone A, a potent antioxidant and effective suppressor of inducible nitric oxide synthase in lipopolysaccharide-activated macrophages. Biochem Pharmacol. 2001;61:939–946. doi: 10.1016/s0006-2952(01)00543-3. [DOI] [PubMed] [Google Scholar]
- Dong H, Chen SX, Xu HX, Kadota S, Namba T. A new antiplatelet diarylheptanoid from Alpinia blepharocalyx. J Natl Prod. 1998;61:142–144. doi: 10.1021/np970293i. [DOI] [PubMed] [Google Scholar]
- Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Struck E, Pahl HL, et al. Cysteine 38 in p65/NFκB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces of the NFκB puzzle. Cell. 2002;109 (Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Herencia F, Ferrandiz ML, Ubeda A, Guillen I, Dominguez JN, Charris JE, et al. Novel anti-inflammatory chalcone derivatives inhibit the induction of nitric oxide synthase and cyclooxygenase-2 in mouse peritoneal macrophages. FEBS Lett. 1999;453:129–134. doi: 10.1016/s0014-5793(99)00707-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yao XQ, Tsang SY, Lau CW, Chen ZY. Role of endothelium/nitric oxide in vascular response to flavonoids and epicatechin. Acta Pharmacol Sin. 2000;21:1119–1124. [PubMed] [Google Scholar]
- Hwang D, Jang BC, Yu G, Boudreau B. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-κB signaling pathways in macrophages. Biochem Pharmacol. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Itokawa H, Morita M, Mihashi S. Phenolic compounds from the rhizomes of Alpinia speciosa. Phytochemistry. 1981;20:2503–2506. [Google Scholar]
- Jayasinghe L, Balasooriya BAIS, Padmini WC, Hara N, Fujimoto N. Geranyl chalcone derivatives with antifungal and radical scavenging properties from the leaves of Artocarpus nobilis. Phytochemistry. 2004;65:1287–1290. doi: 10.1016/j.phytochem.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Kanke T, MacFarlane SR, Seatter MJ, Davenport E, Paul A, McKenzie RC, et al. Proteinase-activated receptor-2-mediated activation of stress-activated protein kinases and inhibitory kappa B kinases in NCTC 2544 keratinocytes. J Biol Chem. 2001;276:31657–31666. doi: 10.1074/jbc.M100377200. [DOI] [PubMed] [Google Scholar]
- Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. 2005;115:375–387. doi: 10.1111/j.1365-2567.2005.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HH, Tsao LT, Yu KL, Liu CT, Wang JP, Lin CN. Structure–activity relationship studies on chalcone derivatives: the potent inhibition of chemical mediators release. Bioorg Med Chem. 2003;11:105–111. doi: 10.1016/s0968-0896(02)00312-7. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- Lahti A, Lahde M, Kankaanranta H, Moilanen E. Inhibition of extracellular signal-regulated kinase suppresses endotoxin-induced nitric oxide synthesis in mouse macrophages and in human colon epithelial cells. J Pharmacol Exp Ther. 2000;294:1188–1194. [PubMed] [Google Scholar]
- Lee JH, Jung HS, Giang PM, Lee S, Jin X, Son PT, et al. Blockade of NF-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analogue from Alpinia conchigera. J Pharmacol Exp Ther. 2006;316:271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- Lee SH, Seo GS, Sohn DH. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem Biophys Res Commun. 2004;323:125–132. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- Liu L, Paul A, MacKenzie CJ, Bryant C, Graham A, Plevin R. Nuclear factor kappa B is involved in lipopolysaccharide-stimulated induction of interferon regulatory factor-1 and GAS/GAF DNA-binding in human umbilical vein endothelial cells. Br J Pharmacol. 2001;134:1629–1638. doi: 10.1038/sj.bjp.0704404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russel SW, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon-γ and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Mertfort I. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB. Biol Chem. 1997;378:951–961. doi: 10.1515/bchm.1997.378.9.951. [DOI] [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Mertfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- MacFarlane SR, Sloss CM, Cameron P, Kanke T, McKenzie RC, Plevin R. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br J Pharmacol. 2005;145:535–544. doi: 10.1038/sj.bjp.0706204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli I, Sebalt A, Bucher C, Charles RP, Nakano H, Doi T, et al. Transient and selective NF-κB p65 Serine 536 phosphorylation induced by T cell costimulation is mediated by IkB kinase β and controls the kinetics of p65 nuclear import. J Immunol. 2004;172:6336–6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Roy MK, Alzoreky NS, Thalang YN, Trakoontivakorn G. Inventory of indigenous plants and minor crops in Thailand based on bioactivities. Ninth JIRCAS International Symposium 2002 – ‘Value-Addition to Agricultural Products'. 2002. pp. 135–139.
- Nielsen SF, Boesen T, Larsen M, Schonning K, Kromann H. Antibacterial chalcones – bioisosteric replacement of the 4-hydroxy group. Bioorg Med Chem. 2004;12:3047–3054. doi: 10.1016/j.bmc.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Paul A, Cuenda A, Murray J, Chilvers ER, Cohen P, Gould GW, et al. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999;11:491–497. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Peng H-B, Libby P, Liao JK. Induction and stabilization of I-κBα by nitric oxide mediates inhibition of NF-κB. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- Rungeler P, Lyss G, Castro V, Mora G, Pahl HL, Mertfort I. Study of three sesquiterpene lactones from Tithonia diversifolia on their anti-inflammatory activity using the transcription factor NF-kappa B and enzymes of the arachidonic acid pathway as targets. Planta Med. 1998;64:588–593. doi: 10.1055/s-2006-957527. [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewtrakul S, Subhadhirasakul S, Puripattanavong J, Panphadung T. HIV-1 protease inhibitory substances from the rhizomes of Boesenbergia pandurata Holtt. Songklanakarin J Sci Technol. 2003;25:503–508. [Google Scholar]
- Trakoontivakorn G, Nakahara K, Shinmoto H, Takenaka M, Onishi-Kameyama M, Ono H, et al. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J Agric Food Chem. 2001;49:3046–3050. doi: 10.1021/jf010016o. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Falvo JV, Tsytsycova AV, Barczak AK, Reimond AM, Glimpser LH, et al. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1 and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. Mol Cell Biol. 2000;16:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi F, Hatano T, Ito H, Yoshida T, Tanuma S. Transcriptional suppression of the HIV promoter by natural compounds. Antiviral Res. 2003;58:89–98. doi: 10.1016/s0166-3542(02)00186-9. [DOI] [PubMed] [Google Scholar]
- Upreti M, Kumar S, Rath PC. Replacement of 198MQMDII203 of mouse IRF-1 by 197IPVEVV202 of human IRF-1 abrogates induction of IFN-beta, iNOS, and COX-2 gene expression by IRF-1. Biochem Biophys Res Commun. 2004;314:737–744. doi: 10.1016/j.bbrc.2003.12.156. [DOI] [PubMed] [Google Scholar]
- Vallejo JGP, Kneuefermann DL, Mann DL, Sivasubramania N. Group B Streptococcus induces TNFα gene expression and activation of the transcription factors NFκB and activator protein-1 in human cord blood monocytes. Infect Immun. 2000;58:808–815. doi: 10.4049/jimmunol.165.1.419. [DOI] [PubMed] [Google Scholar]
- Viana GSB, Bandeira MAM, Matos FJA. Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva Allemão. Phytomedicine. 2003;10:189–195. doi: 10.1078/094471103321659924. [DOI] [PubMed] [Google Scholar]
- Wang D, Baldwin AS., Jr Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on Serine 529. J Biol Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Lau CW, Chan FL, Yao X, Chen ZY, He ZD, et al. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J Cardiovasc Pharmacol. 2001;37:596–606. doi: 10.1097/00005344-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Won SJ, Liu CT, Tsao LT, Weng JR, Ko HH, Wang JP, et al. Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. Eur J Med Chem. 2005;40:103–112. doi: 10.1016/j.ejmech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Xaragori A, Roussos C, Papapetropoulos A. Inhibition of LPS- stimulated pathways in macrophages by the flavonoid luteolin. Br J Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-Kb/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]