Abstract
Background and purpose:
ATP-sensitive K+ (KATP) channels are composed of pore-forming subunits (Kir6.x) and of sulphonylurea receptors (SUR). Both sulphonylureas and KATP channel openers act by binding to SUR. Sulphonylureas reach their binding site from the cytosol but it remains unknown whether this holds for openers too.
Experimental approach:
A poorly membrane-permeant sulphonic acid derivative of the benzopyran-type opener, bimakalim, was synthesized, descyano-bimakalim-6-sulphonic acid (BMSA). Binding of BMSA and bimakalim was compared in membranes and intact cells expressing the Kir6.2/SUR2B channel and channel opening was compared in inside-out patches and whole cells.
Key results:
In membranes, bimakalim and BMSA bound to Kir6.2/SUR2B with Ki values of 61 nM and 4.3 μM, showing that the negative charge decreased affinity 69-fold. In intact cells, however, binding of BMSA was much weaker than in membranes (75-fold) whereas that of bimakalim was unchanged. The Ki value of BMSA decreased with increasing incubation time. In inside-out patches, bimakalim (1 μM) and BMSA (100 μM) opened the Kir6.2/SUR2B channel closed by MgATP to a similar degree whereas in whole-cell experiments, only bimakalim was effective.
Conclusions and implications:
Despite its negative charge, BMSA is an effective channel opener. The fact that BMSA binds and acts more effectively when applied to the inner side of the cell membrane shows that benzopyran openers reach their binding site at SUR from the cytosol. This suggests that the binding pocket of SUR is only open on the cytoplasmic side.
Keywords: KATP channel openers, bimakalim sulphonic acid, access to binding site, sidedness of opener effect, sulphonylurea receptor
Introduction
ATP-sensitive K+ channels (KATP channels) are the targets of synthetic drugs such as the sulphonylureas, which close the channel and the channel openers. The sulphonylureas, exemplified by glibenclamide, close the KATP channel preferably in pancreatic β-cells thereby promoting insulin secretion. The openers are a heterogeneous group of compounds, which have been assessed in therapeutic areas such as hypertension, irritable bladder, asthma and cardiac ischaemia; prominent examples are the benzoypyrans like bimakalim and the cyanoguanidines like P1075 (Ashcroft and Ashcroft, 1990; Coghlan et al., 2001; Mannhold, 2004).
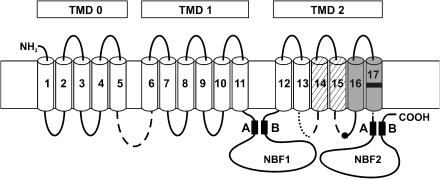
KATP channels are composed of two types of subunits, an inwardly rectifying K+ channel (Kir)6.x, which forms the pore, and sulphonylurea receptors (SURs), which serve as regulatory subunits (see for reviews: Seino and Miki, 2003; Bryan et al., 2005). SURs are members of the ATP-binding cassette (ABC) proteins and comprise three transmembrane domains (TMDs), numbered TMD0 through TMD2, and two intracellular nucleotide-binding folds (Figure 1). In addition, SUR provides the binding sites for sulphonylureas and openers (Hambrock et al., 1998, 2001; Schwanstecher et al., 1998; Figure 1). Subtypes of Kir and SUR exist and are differentially expressed in different tissues (Seino and Miki, 2003).
Figure 1.
SUR and the binding sites for openers and sulphonylureas. The transmembrane topology of SUR is according to Tusnády et al. (1997) and shows the organization of the 17 transmembrane segments in three TMDs, TMD0–TMD2. In the two nucleotide binding folds, the Walker A and B motifs are indicated. The binding site for the benzopyran and cyanoguanidine openers is formed by segments 16 and 17 and by a part (dotted) of the cytosolic loop linking segments 13 and 14 (Uhde et al., 1999). The black mark in segment 17 gives the approximate location of M1290 (SUR1) or T1253 (SUR2). The binding site of glibenclamide lies in segments 14 and 15 (hatched) and parts of the associated cytosolic loops, and in the loop linking TMD0 and TMD1 (broken lines). The black dot in the middle of the loop linking segments 15 and 16 represents S1237/Y1206 in SUR1/SUR2, which is of great importance for glibenclamide binding (Ashfield et al., 1999).
The binding site for glibenclamide at SUR is composed of transmembrane segments 14 and 15 and parts of the connecting cytosolic loops, and of the cytosolic loop, which links TMD0 and TMD1 (Ashfield et al., 1999; Uhde et al., 1999; Mikhailov et al., 2001; see Figure 1). The binding site for benzopyran and cyanoguanidine openers is formed by transmembrane segments 16 and 17 in TMD2 and part of the cytosolic loop linking segments 13 and 14 (Uhde et al., 1999; see Figure 1). Residues L1249 and T1253 (SUR2, rat numbering), located in the lower part of the upper half of segment 17, have been shown to be crucial for the sensitivity of the channel to benzopyran- and cyanoguanidine-type openers (Moreau et al., 2000). In SUR1, M1290 corresponds to T1253 in SUR2, and the importance of this amino acid for the binding of openers to SUR1 was demonstrated in radioligand-binding studies (Hambrock et al., 2004). Extensive mutational analysis suggested that this residue acted as a gate to control the access of benzopyran- and cyanoguanidine-type openers to their binding site (Moreau et al., 2005a), and it was proposed that these openers access their binding site from the outside (Moreau et al., 2005b).
The pathway by which a compound reaches its site on a membrane protein is often explored by applying a permanently charged, membrane-impermeant derivative to either side of the membrane and comparing the effects. This approach was successfully applied to clarify the access of local anaesthetics to their site at the Na+ channel (see e.g. Hille, 1977), and of the phenylalkylamines to the Ca2+ channel (Hescheler et al., 1982). Similarly, Schwanstecher et al. (1994) prepared a poorly membrane-permeant sulphonic acid derivative of meglitinide, which was much more powerful when applied to the inside than to the outside of the cell membrane; the authors concluded that sulphonylureas reach their binding site at SUR from the cytosol. Regarding the openers, such experiments had not yet been carried out, because a permanently charged derivative was not available so far. The proposal that openers access their binding site from the outside (Moreau et al., 2005b) has to be interpreted in the context that the binding sites for the openers and the sulphonylureas are located in neighbouring regions of SUR (Figure 1) and that they are strongly coupled by negative allosteric interactions (Bray and Quast, 1992; Schwanstecher et al., 1992; Löffler-Walz et al., 2002). In the light of these observations, it would be intriguing if the opener binding site was reached from the outside and the sulphonylurea site from the inside, and we sought to obtain independent evidence for the route by which the openers access their binding site.
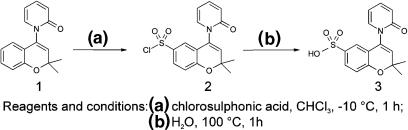
Adopting the strategy outlined above, a negatively charged analogue of the benzopyran opener, bimakalim, was synthesized by replacing the cyano-substituent in position 6 with sulphonic acid (descyano-bimakalim sulphonic acid (BMSA); Figure 2). Hoping that BMSA still acted as an effective opener, binding of BMSA in membranes was compared with that in intact cells, and channel opening by BMSA in inside-out patches was compared with that in whole cells. The cell-permeant parent compound, bimakalim, was used for comparison. The KATP channel, Kir6.2/SUR2B (i.e. the channel predominant in non-vascular smooth muscle), was selected, as SUR2B has the highest affinity for benzopyran-type openers (Hambrock et al., 1999), and this channel is easily studied in isolated patches.
Figure 2.
Synthesis of BMSA. 1, descyano-bimakalim; 2, descyano-bimakalim-6-sulphochloride; 3, BMSA (see Materials for details). The pKa value of benzosulphonic acid is 0.7 (Weast, 1981–1982). Assuming that this holds also for BMSA, at pH 7.4, the ratio of the charged to the neutral form is 107.4–0.7=5 × 106.
Materials and methods
Cell culture, transfection and membrane preparation
Human embryonic kidney (HEK) 293 cells were cultured in minimum essential medium (MEM) containing glutamine and supplemented with 10% fetal bovine serum and 20 μg ml−1 gentamycin as described by Hambrock et al. (1998). For transient expression of the Kir6.2/SUR2B channel, cells were transfected with the murine clones (GenBank accession numbers D86038 and D50581) incorporated in pcDNA 3.1 vectors (Invitrogen, Karlsruhe, Germany). Lipofectamine 2000 and Opti-MEM (Invitrogen) were used as transfection reagents according to the manufacturer's instructions. For electrophysiological studies, pEGFP-C1 vector (Clontech, Palo Alto, CA, USA), encoding for green fluorescent protein, was added for easy identification of transfected cells. Cells were used for membrane preparation 2–4 days after transfection. A crude membrane fraction was prepared as described by Hambrock et al. (1998) and frozen at −80°C in Mg2+-free incubation buffer containing (in mM) 4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid (HEPES) 5, NaCl 139 and KCl 5 and adjusted to pH 7.4 at 4°C. Protein concentration was determined according to Lowry et al. (1951) using bovine serum albumin as the standard.
[3H]P1075 competition experiments
Binding experiments in intact cells and in membranes were performed as described by Hambrock et al. (2001). Briefly, an incubation buffer was prepared containing (in mM) NaCl 129, KCl 5, MgCl2 1.2, CaCl2 1.25, D(+)-glucose 11, NaHCO3 5, HEPES 10, gassed with O2 at pH 7.4. The buffer was supplemented with the opener [3H]P1075 (∼2 nM) as the radioligand and the competing openers BMSA or bimakalim, and incubation was started by the addition of membranes or cells to give 1 ml final solution. After 30 or 120 min, incubation was stopped by diluting 0.3 ml aliquots in triplicate into 8 ml of ice-cold quench solution (50 mM tris-(hydroxymethyl)-aminomethane, 154 mM NaCl, pH 7.4) and rapid filtration under vacuum over Whatman GF/C (cells) or GF/B filters (membranes). Filters were washed twice with quench solution and counted for 3 h in the presence of 4.5 ml of scintillant (Ultima Gold; Packard, Meriden, CT, USA). Nonspecific binding was determined in the presence of 100 μM unlabelled P1075 and was between 10 and 30% of total binding.
Patch-clamp experiments
Patch-clamp experiments in the inside-out configuration were performed as described by Russ et al. (2001). Bath and pipette were filled with a high-K+ Ringer solution containing (in mM) KCl 142, NaCl 2.8, MgCl2 1, CaCl2 1, D(+)-glucose 11, HEPES 10, titrated to pH 7.4 with NaOH at 22°C. After filling with buffer, pipettes had a resistance of 0.8–1.0 MΩ. After excision of the patch, the pipette was moved in front of a pipe with a high-K+ solution containing (in mM) KCl 143, MgCl2 0.85, CaCl2 1, ethylene glycol-bis-(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 5, D(+)-glucose 11, HEPES 10, titrated to pH 7.2 with NaOH at 22°C. KATP channel modulators and ATP (free Mg2+ was kept at 0.7 mM) were dissolved as described below and added to the pipe solution. Patches were clamped to −50 mV. The difference in current in the absence and presence of MgATP (1 mM) was taken as the current passing through KATP channels (IKATP) and the effect of openers, applied in the presence of MgATP, was expressed as % IKATP.
Experiments using the cell-attached configuration were performed under the same conditions as the inside-out experiments but at 37°C (composition of pipette and bath buffer as above, pipette resistance 0.8–1 MΩ, holding potential −50 mV).
Experiments in the whole-cell configuration were performed as described by Russ et al. (1999). The bath was filled with (in mM) NaCl 142, KCl 2.8, MgCl2 1, CaCl2 1, D(+)-glucose 11, HEPES 10, pH 7.4 at 22 or 37°C, and patch pipettes were filled with (in mM) K-glutamate 126, NaCl 10, MgCl2 4, HEPES 10, EGTA 1 and Na2ATP 3, pH 7.2, and had a resistance of 3–5 MΩ. Cells were clamped to −60 mV. Every 12 s, seven square pulses ranging from −110 to 10 mV and lasting 0.5 s each were applied, which allows one to construct a current–voltage curve at any moment and to assure the quality of the experiment (not shown). Series resistance was compensated by 70%.
Data were recorded with an EPC 9 amplifier (HEKA, Lambrecht, Germany) using the ‘Pulse' software (HEKA). Signals were filtered at 200 Hz using the four-pole Bessel filter of the EPC9 amplifier and sampled with 1 kHz.
Data analysis
Binding inhibition curves were analysed according to the equation
as described by Hambrock et al. (1998) with y denoting specific binding, x the inhibitor concentration with px=−log x and IC50 the inhibitor concentration required for 50% inhibition with pIC50=−log IC50. IC50 values were converted into inhibition constants (Ki) by correcting for the presence of the radioligand ([3H]P1075) according to the equation of Cheng and Prusoff (1973).
with [L] denoting the concentration of [3H]P1075 (∼2 nM) and KD the equilibrium dissociation constant (4 nM), giving a correction factor ∼1.5. Individual binding curves were analysed and the pKi values were pooled assuming that they are normally distributed (Christopoulos, 1998). In the text, Ki values are given with their 95% confidence interval.
The time course of the electrophysiological traces was analysed by fitting an exponential function to them to determine half-times and amplitudes. All calculations were performed using Sigmaplot 6.1 (SPSS Science, Chicago, IL, USA).
Materials
BMSA was synthesized via the route shown in Figure 2. Compounds 1 and 2 were synthesized as described by Mannhold et al. (1999) and Salamon et al. (2002). The sulphochloride (compound 2) was mixed with water and refluxed for 1 h. After cooling, the aqueous phase was extracted with chloroform and evaporated to dryness. The residue was recrystallized from methanol/ether to give colourless crystals with melting point 284–286°C. The purity of the final product was analysed by 1H-NMR spectroscopy and elemental analysis; the results of the latter were within 0.3% of the theoretical values.
[3H]P1075 with a specific activity of 4.5 TBq/mmol was purchased from Amersham Buchler (Braunschweig, Germany). Bimakalim was a kind gift from Merck Pharma (Darmstadt, Germany) and P1075 from Leo Pharmaceuticals (Ballerup, Denmark). BMSA (30 mM stock concentration) was dissolved in equimolar NaOH or in HEPES (0.1 M) and the other KATP channel modulators in dimethyl sulphoxide/ethanol (50/50, v/v) and further diluted with the same solvent or with incubation buffer (final solvent concentration in the assays <1%).
Results
Binding of BMSA
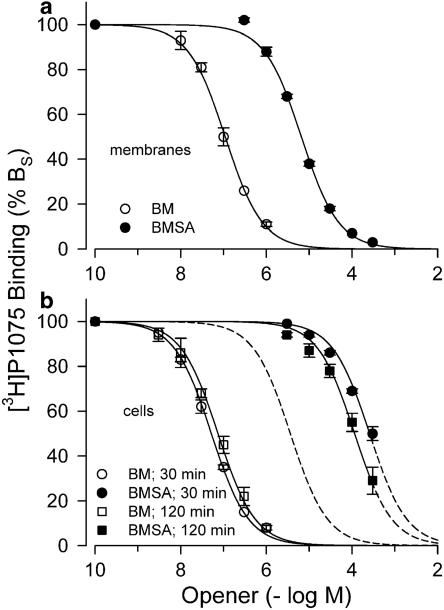
Figure 3a shows the inhibition of [3H]P1075 binding to Kir6.2/SUR2B by bimakalim and BMSA in membranes. Both inhibition curves were regular with Hill coefficient 1, reached completion and gave Ki values of 0.061 and 4.3 μM for bimakalim and BMSA, respectively. This showed that replacement of the cyano group in position 6 of the benzopyran ring by the negatively charged sulphonic acid group (Figure 2) reduced binding affinity 69 times (Table 1).
Figure 3.
Binding of bimakalim (BM) and BMSA to Kir6.2/SUR2B as determined by inhibition of [3H]P1075 binding. (a) Experiments in membranes; incubation time was 30 min. (b) Experiments in intact cells; incubation times were 30 and 120 min. Data are means from 3 to 4 experiments giving the Ki values listed in Table 1. The broken curve illustrates the theoretical binding curve for BMSA, if the binding site was freely accessible, that is, shifted to the right from the BM curve measured at 30 min by a factor of 69. Experiments were performed at 37°C; [3H]P1075 concentration was ∼2 nM.
Table 1.
Binding of BM and BMSA to SUR2B in membranes and intact cells
| Preparation | Incubation time (min) | Ki (BM) (nM) | Ki (BMSA) (μM) | Ki (BMSA)/Ki (BM)a |
|---|---|---|---|---|
| Kir6.2/SUR2B (membranes) | 30 | 61 (51, 74) | 4.3 (4.1, 4.5) | 69 (58, 83) |
| Kir6.2/SUR2B (cells) | 30 | 35 (34, 37) | 186 (162, 214) | 5200 (4600, 6000) |
| 120 | 52 (45, 62)* | 85 (59, 123)* | 1600 (1100, 2400)* | |
| SUR2B alone (cells) | 30 | 44 (42, 46) | 169 (147, 193) | 3900 (3400, 4500) |
Abbreviations: BM, bimakalim; BMSA, descyano-bimakalim-6-sulphonic acid; Kir, inwardly rectifying K+ channel; SUR, sulphonylurea receptor.
Binding inhibition curves are shown in Figure 3.
Ki values are followed by the 95% confidence interval.
Significantly different from the respective value at 30 min (P<0.05, t-test performed at the level of the pKi values).
Ratio calculated from the difference of the pKi values taking propagation of errors into account.
Figure 3b illustrates binding experiments in intact cells with incubation times of 30 and 120 min. At 30 min, binding of bimakalim was similar to that in membranes; in contrast, BMSA bound with very weak affinity (Table 1). Hence, in intact cells, the ratio of the Ki values of BMSA over bimakalim was 5200, far above the value determined in membranes (Table 1). After 120 min incubation time, the Ki value of bimakalim was slightly increased and that of BMSA decreased, giving a ratio of 1600 (Table 1).
In addition, binding experiments with intact cells expressing SUR2B alone were performed. SUR alone exposes a retention motif (RKR) that retains the protein in the endoplasmic reticulum; in the Kir/SUR complex, the retention motif is masked, allowing transport of the assembled channel to the cell membrane (Zerangue et al., 1999). Table 1 shows that the affinity of bimakalim binding to SUR2B expressed alone was very similar to that for the assembled channel. This showed that coexpression with Kir6.2 did not change the affinity of SUR2B for openers (see also Hambrock et al., 2001). BMSA binding to SUR in intact cells was again very weak and not different from that to the assembled channel (Table 1).
BMSA as a channel opener
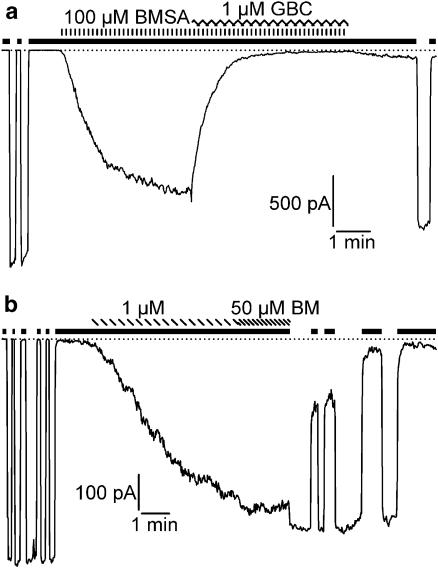
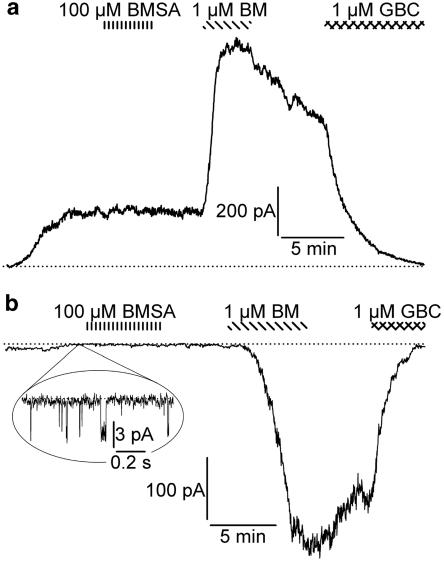
The channel opening properties of BMSA and BM were compared in several configurations of the patch-clamp technique at 22 and 37°C. The concentration–response curve for channel opening generally lies to the right of the opener binding curve by factors ranging from 5 (Schwanstecher et al., 1998) to 10 (Quast et al., 1993); therefore, concentrations of about 10 to 20 × Ki were chosen, that is, BMSA 100 μM, bimakalim 1 μM. Figure 4 shows experiments using inside-out patches at 22°C with drug application to the bath, that is, to the cytoplasmic face of the patch. BMSA (100 μM) induced an inward current that was completely reversed by glibenclamide (1 μM) (Figure 4a). Figure 4b shows the current induced by bimakalim (1 μM); in this experiment, 50 μM bimakalim produced no further effect. Table 2 summarizes the results from 5 patches, showing that in the inside-out configuration, BMSA (100 μM) induced a glibenclamide-sensitive current of the same magnitude as bimakalim at the saturating concentration of 50 μM. In whole-cell measurements at 37°C (Figure 5a), bath application of BMSA (100 μM, 5 min) was ineffective, whereas, in the same cell, bimakalim (1 μM) induced a rapid and strong response that was completely reversed by glibenclamide (1 μM). These results were confirmed in four cells (Table 2). In addition, experiments were performed in the cell-attached mode with high K+ in the pipette (Figure 5b and Table 2). Again, BMSA, applied to the bath for 6 min, was ineffective, whereas bimakalim elicited a large glibenclamide-sensitive current.
Figure 4.
Original traces showing BMSA and bimakalim (BM) as openers of the Kir6.2/SUR2B channel in inside-out patches. Patches were exposed to symmetrical high-K+ solution at 22°C and clamped at −50 mV. The black bars indicate the application of MgATP (1 mM) and the dotted lines the zero current level. Initial intermittent application of MgATP defined the 100% level of the ATP-inhibited current, IKATP. (a) BMSA (100 μM) induced an inward current that was abolished by glibenclamide (GBC, 1 μM). (b) Effect of bimakalim (1 and 50 μM). For evaluation of the currents see Table 2.
Table 2.
Channel opening effect of BMSA and bimakalim
| Condition | Opener | IKCO (% IKATP or pA)a | t1/2 (min) |
|---|---|---|---|
| i/o (22°C) | BMSA | 72±11% | 0.4±0.1 |
| BM | 57±7% | 1.8±0.1 | |
| BM (50 μM) | 81±4% | 0.10±0.02 | |
| wc (22°C) | BMSA | 0 | — |
| BM | 440±220b pA | 2.1±0.2 | |
| wc (37°C) | BMSA | 0 | — |
| BM | 570±180b pA | 0.4±0.1 | |
| On cell (37°C) | BMSA | 0 | — |
| BM | 490±130 pA | 1.4±0.1 |
Abbreviations: BM, bimakalim; BMSA, descyano-bimakalim-6-sulphonic acid i/o, inside-out; wc, whole- cell, on cell, cell-attached; IKCO denotes the opener-induced current.
Experiments were performed as shown in Figures 4 and 5. BMSA concentration was 100 μM and BM 1 μM if not stated otherwise. Data are means±s.e.m. of 4–7 experiments.
In the experiments using the inside-out configuration, IKCO was expressed as % of the current in the absence of MgATP (% IKATP); in the other experiments, as pA.
Basal (dialysis-induced) currents of 11 pA (22°C, n=4) or 110±21 pA (37°C, n=6) were subtracted.
Figure 5.
Effect of BMSA (100 μM) and bimakalim (BM; 1 μM) on the Kir6.2/SUR2B channel in the whole-cell and cell-attached configurations. (a) Whole-cell configuration at 37°C. The cell was bathed in a physiological K+ Ringer solution and clamped at −60 mV. Dialysis of the cell with MgATP (3 mM) in a high-K+ Ringer induced a current of ∼200 pA. Superfusion with BMSA (100 μM, 5 min) had no effect, whereas bimakalim (1 μM) rapidly produced a large current. Application of glibenclamide (1 μM) during the washout phase restored the zero current level. (b) Cell-attached configuration at 37°C. Bath and pipette were filled with a high-K+ Ringer solution and the patch clamped at −50 mV. Application of BMSA (100 μM, 6 min) to the bath produced no current, whereas bimakalim (1 μM) induced a large inward current, which was rapidly abolished by glibenclamide (1 μM) superfused during washout. The close-up before the superfusion of BMSA shows spontaneous single channel openings giving rise to currents of ∼4 pA, in agreement with the single channel conductance of Kir6.2 (80 pS in symmetrical high K+) at −50 mV (Seino and Miki, 2003). For means of relevant parameters, see Table 2.
Surprisingly, the channel opening kinetics effects of bimakalim (1 μM) were slow in the inside-out and rapid in the whole-cell configuration (Figures 4b and 5a, and Table 2). In order to examine whether this difference was owing to the different temperatures at which the two sets of experiments were performed (22 vs 37°C), whole-cell experiments with bimakalim were also performed at 22°C. Table 2 shows that under these conditions, the opening kinetics of bimakalim were as slow as in the inside-out configuration (half-times ∼2 min).
Discussion
In this study, we sought to clarify the pathway by which benzopyran-type KATP channel openers reach their binding site in the membrane. Following the strategy outlined in Introduction, a highly polar opener was synthesized by replacing the cyano-substituent of bimakalim with sulphonic acid. To be a useful tool, this compound had to meet two requirements: first, it should be functionally active and, second (essentially) membrane-impermeant.
Regarding activity, radioligand-binding experiments with BMSA in membranes (where the binding site is freely accessible) showed that the introduction of the negative charge reduced the affinity to Kir6.2/SUR2B by a factor of 69. In addition, when applied to the cytoplasmic side of inside-out patches, the compound exhibited an efficacy in opening the Kir6.2/SUR2B channel similar to that of the parent compound, bimakalim. Hence, BMSA was an effective opener, albeit with reduced affinity. Regarding membrane permeation, BMSA bound with very low affinity to SUR located intracellularly (Table 1), that is, to intact cells expressing SUR2B alone (Zerangue et al., 1999). In these cells, the affinity of BMSA was much lower than in membranes, whereas that of bimakalim was similar in the two preparations (Table 1). This showed that BMSA did not permeate the membrane easily. Hence, the new probe met the two requirements. In addition, the inactivity of BMSA in the whole-cell and cell-attached configurations of the patch-clamp technique suggested that the BMSA preparation was ‘pure', that is, devoid of small (∼1%) contaminations of membrane-permeant active species such as ‘descyano-bimakalim' (Figure 2).
What can be inferred from the finding that BMSA exhibited much higher affinity to Kir6.2/SUR2B in membranes than in intact cells and that it activated the channel if applied to the cytoplasmic face of inside-out patches, but not if applied to the extracellular side of intact cells? Apparently, on the short time scale of the electrophysiological experiments (application time <10 min), BMSA did not reach its binding site in sufficient amounts; after 30 min, however, the concentration was high enough to be detected by the more sensitive binding assay. This showed that the poorly membrane-permeant BMSA reached its binding site only very slowly if applied to the outside of the cell. It is therefore concluded that benzopyran-type openers access their binding site at SUR from the cytosol. In contrast to BMSA, bimakalim bound and acted equally well from either side of the membrane, showing that this compound easily diffuses through the membrane.
Some information on the ability of BMSA to cross the cell membrane was obtained from the whole-cell binding experiments. The large Ki values in these experiments reflect the fact that the inhibition curves are based on the BMSA concentrations present in the incubation solution, whereas only a small fraction thereof had yet been able to enter the cell and to reach the binding site. Therefore, these Ki values are apparent Ki values, and they decrease as the concentration of BMSA in the cell increases slowly with time (Table 1). One may therefore consider the change of the ratio Ki(BMSA)/Ki(BM) with time as a measure of the fraction of BMSA that has entered the cell. At equilibrium, this ratio will reach the value observed in membranes (69), and after 2 h, cell entry is 69/1600=4.3% complete (Table 1). Assuming an exponential time course, a half-time of ∼30 h is then estimated for the process (not shown). This surprisingly long half-time is a direct consequence of the highly acidic nature of BMSA as, at pH 7.4, only one out of 5 × 106 molecules is in the uncharged form (see legend to Figure 2). Assuming that BMSA enters the cell by diffusion and that only the uncharged form is membrane-permeant, this would lead to a half-time of about 30 h/(5 × 106)=20 ms for the uncharged form to cross the membrane and to reach its binding site. Further, setting the length of the path for crossing the membrane and reaching the binding to 100 Å would yield a diffusion coefficient of 3.5 × 107 cm2 s−1, which compares well with that for glycine in H2O at 20°C (D=9.4 × 106 cm2 s−1; van Holde, 1971).
SURs are members of the ABC transporter superfamily. It is unknown whether SUR can act as a transporter; however, one cannot exclude the possibility that SUR may transport BMSA applied to the outside into the cell. This possibility is, however, very unlikely, because, in whole cells we found similar binding of BMSA to Kir6.2/SUR2B (which is located in the membrane and could eventually transport into the cell) and to SUR2B alone (which is located intracellularly and cannot transport). Hence, diffusion is the favoured mechanism of entry.
Finally, we would like to consider how the conclusion drawn here, that is, that benzopyran-type openers reach their binding site at SUR from the cytosol, fits with the known structural features of SUR. First, we note that this conclusion agrees with that of Schwanstecher et al. (1994) that sulphonylureas act on the pancreatic KATP channel from the cytosol; this study was based on experiments with a sulphonic acid derivative of meglitinide. In view of the physical proximity of the binding sites for non-diazoxide openers and sulphonylureas (Figure 1; Ashfield et al., 1999; Uhde et al., 1999; Moreau et al., 2005a), this agreement appears plausible. Based on the structures of bacterial ABC transporters, homology modelling suggests that SUR contains a large binding chamber of triangular shape with the base and lower part formed by cytosolic loops of TMD1 and 2 and the sides by transmembrane segments of TMD1 and 2 (Bryan et al., 2004, 2005). The residue T1253 (SUR2) or M1290 (SUR1), located in the middle of transmembrane segment 17, may be at (or close to) the top of the chamber (Bryan et al., 2004). The results obtained here are difficult to reconcile with the proposition of Moreau et al. (2005a), Moreau et al. (2005b) that M1290 (SUR1) forms the access gate for benzopyran openers from the outside. It is, however, entirely possible that M1290 in SUR 1 obstructs binding (Hambrock et al., 2004) and effect (Moreau et al., 2005a, 2005b) of benzopyran- and cyanoguanidine-type openers in the chamber either by sterical hindrance or by allosteric distortion of the pocket.
In conclusion, we have shown here that a poorly membrane-permeant sulphonic acid derivative of bimakalim binds to and opens the Kir6.2/SUR2B channel much more weakly when applied to the outside, than to the inside, of the cell. This suggests that benzopyran-type KATP channel openers reach their binding site from the cytosol.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Qu 100/3-2). We thank Drs Y Kurachi and Y Horio (Osaka) for the generous gift of the murine clones of SUR2B and Kir6.2 and Mrs Magdalene Matyja (Pharmaceutical Chemistry, Düsseldorf) for the excellent technical assistance with the synthesis of BMSA. We gratefully acknowledge fruitful discussions with Marcus Winkler, Tübingen.
Abbreviations
- BMSA
descyano-bimakalim-6-sulphonic acid
- HEK cells
human embryonic kidney 293 cells
- HEPES
4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid
- KATP channels
ATP-sensitive K+ channel
- Ki
inhibition constant
- Kir
inwardly rectifying K+ channel
- P1075
N-cyano-N′-(1,1-dimethylpropyl)-N″-3-pyridylguanidine
- SUR
sulphonylurea receptor
- TMD
transmembrane domain
Conflict of interest
The authors state no conflict of interest.
References
- Ashcroft SJH, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashfield R, Gribble FM, Ashcroft SJH, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- Bray KM, Quast U. A specific binding site for K+ channel openers in rat aorta. J Biol Chem. 1992;267:11689–11692. [PubMed] [Google Scholar]
- Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and KATP channels. Curr Pharm Design. 2005;11:2699–2716. doi: 10.2174/1381612054546879. [DOI] [PubMed] [Google Scholar]
- Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes. 2004;53:S104–S112. doi: 10.2337/diabetes.53.suppl_3.s104. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Assessing the distribution of parameters in models of ligand-receptor interaction: to log or not to log. Trends Pharmacol Sci. 1998;19:351–357. doi: 10.1016/s0165-6147(98)01240-1. [DOI] [PubMed] [Google Scholar]
- Coghlan MJ, Carroll WA, Gopalakrishnan M. Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J Med Chem. 2001;44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- Hambrock A, Kayar T, Stumpp D, Osswald H. Effect of two amino acids in TM17 of sulfonylurea receptor SUR1 on the binding of ATP-sensitive K+ channel modulators. Diabetes. 2004;53:S128–S134. doi: 10.2337/diabetes.53.suppl_3.s128. [DOI] [PubMed] [Google Scholar]
- Hambrock A, Löffler-Walz C, Kloor D, Delabar U, Horio Y, Kurachi Y, et al. ATP-Sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol Pharmacol. 1999;55:832–840. [PubMed] [Google Scholar]
- Hambrock A, Löffler-Walz C, Kurachi Y, Quast U. Mg2+ and ATP dependence of KATP channel modulator binding to the recombinant sulphonylurea receptor, SUR2B. Br J Pharmacol. 1998;125:577–583. doi: 10.1038/sj.bjp.0702109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrock A, Löffler-Walz C, Russ U, Lange U, Quast U. Characterization of a mutant sulfonylurea receptor SUR2B with high affinity for sulfonylureas and openers: differences in the coupling to Kir6.x subtypes. Mol Pharmacol. 2001;60:190–199. doi: 10.1124/mol.60.1.190. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Pelzer D, Trube G, Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflügers Arch-Eur J Physiol. 1982;393:287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug–receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler-Walz C, Hambrock A, Quast U. Interaction of KATP channel modulators with sulfonylurea receptor SUR2B: implication for tetramer formation and allosteric coupling of subunits. Mol Pharmacol. 2002;61:407–414. doi: 10.1124/mol.61.2.407. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mannhold R. KATP channel openers: structure–activity relationships and therapeutic potential. Med Sci Res. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- Mannhold R, Cruciani G, Weber H, Lemoine H, Derix A, Weichel C, et al. 6-Substituted benzopyrans as potassium channel activators: synthesis, vasodilator properties, and multivariate analysis. J Med Chem. 1999;42:981–991. doi: 10.1021/jm981047m. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Mikhailova EA, Ashcroft SJH. Molecular structure of the glibenclamide binding site of the β-cell K-ATP channel. FEBS Lett. 2001;499:154–160. doi: 10.1016/s0014-5793(01)02538-8. [DOI] [PubMed] [Google Scholar]
- Moreau C, Gally F, Jacquet-Bouix H, Vivaudou M. The size of a single residue of the sulfonylurea receptor dictates the effectiveness of KATP channel openers. Mol Pharmacol. 2005a;67:1026–1033. doi: 10.1124/mol.104.008698. [DOI] [PubMed] [Google Scholar]
- Moreau C, Jacquet H, Prost A-L, D'hahan N, Vivaudou M. The molecular basis of the specificity of action of KATP channel openers. EMBO J. 2000;19:6644–6651. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Prost A-L, Dérand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005b;38:951–963. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Quast U, Bray KM, Andres H, Manley PW, Baumlin Y, Dosogne J. Binding of the K+ channel opener [3H]P1075 in rat isolated aorta: relationship to functional effects of openers and blockers. Mol Pharmacol. 1993;43:474–481. [PubMed] [Google Scholar]
- Russ U, Hambrock A, Artunc F, Löffler-Walz C, Horio Y, Kurachi Y, et al. Coexpression with the inward rectifier K+ channel Kir6.1 increases the affinity of the vascular sulfonylurea receptor SUR2B for glibenclamide. Mol Pharmacol. 1999;56:955–961. [PubMed] [Google Scholar]
- Russ U, Lange U, Löffler-Walz C, Hambrock A, Quast U. Interaction of the sulfonylthiourea HMR 1883 with sulfonylurea receptors and recombinant ATP-sensitive K+ channels: comparison with glibenclamide. J Pharmacol Exp Ther. 2001;299:1049–1055. [PubMed] [Google Scholar]
- Salamon E, Mannhold R, Weber H, Lemoine H, Frank W. 6-Sulfonylchromenes as highly potent KATP-channel openers. J Med Chem. 2002;45:1086–1097. doi: 10.1021/jm010999g. [DOI] [PubMed] [Google Scholar]
- Schwanstecher M, Brandt C, Behrends S, Schaupp U, Panten U. Effect of MgATP on pinacidil-induced displacement of glibenclamide from the sulphonylurea receptor in a pancreatic β-cell line and rat cerebral cortex. Br J Pharmacol. 1992;106:295–301. doi: 10.1111/j.1476-5381.1992.tb14331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher M, Schwanstecher C, Dickel C, Chudziak F, Moshiri A, Panten U. Location of the sulphonylurea receptor at the cytoplasmic face of the β-cell membrane. Br J Pharmacol. 1994;113:903–911. doi: 10.1111/j.1476-5381.1994.tb17078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanstecher M, Sieverding C, Dörschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Tusnády GE, Bakos E, Varadi A, Sarkadi B. Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Lett. 1997;402:1–3. doi: 10.1016/s0014-5793(96)01478-0. [DOI] [PubMed] [Google Scholar]
- Uhde I, Toman A, Gross I, Schwanstecher C, Schwanstecher M. Identification of the potassium channel opener site on sulfonylurea receptors. J Biol Chem. 1999;274:28079–28082. doi: 10.1074/jbc.274.40.28079. [DOI] [PubMed] [Google Scholar]
- Van Holde KE. Physical Biochemistry. Prentice-Hall Inc.: Englewood Cliffs, NJ; 1971. p. 89. [Google Scholar]
- Weast RE. CRC Handbook of Chemistry and Physics 1981–1982CRC Press Inc.: Cleveland, OH; (ed).Table D-142 Dissociation constants of organic acids in aqueous solution 62nd edn [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]